
Phytochemical Compositions and Cosmeceutical Activities of Rosa damascena Mill. Leaf Extracts from Environmentally Friendly Extraction Technique
Pipat Jittasai, Watchara Kanjanakawinkul, Artit Yawootti and Wantida Chaiyana*Published Date : January 5, 2024
DOI : https://doi.org/10.12982/NLSC.2024.008
Journal Issues : Number 1, January-March 2024
Abstract A significant amount of Rosa damascena Mill. leaves were discarded as waste during the harvesting and pruning for flowering. The current study aimed to investigate the potential of using these agricultural waste products in the cosmetics industry. Dried R. damascena leaves were extracted using maceration or environmentally friendly extractions, including infusion, digestion, ultrasonic, microwave, micellar, and pulsed electric fields (PEF) extraction. The extracts were analyzed for their chemical compositions and assessed for antioxidant, anti-tyrosinase, and anti-aging properties. The irritation profile of each extract was investigated by the hen’s egg-chorioallantoic membrane (HET-CAM) test. The results noted that extraction by ethanol yielded significantly higher extract content than deionized water (P < 0.05). However, total phenolic and flavonoid content was found to be greater, whereas the bioactive compounds, including rutin and rosmarinic acid, were only found in the aqueous extracts. On the other hand, kojic acid was found in the extract from infusion and maceration. Interestingly, the extracts from micellar and PEF extractions were found to significantly scavenge the radical, which were related to their rutin contents. Their collagenase inhibition (69.1 ± 10.1% and 54.8 ± 19.1%, respectively) were equivalent to those of epigallocatechin gallate, a well-known anti-aging compound (76.0 ± 1.2%). Besides, all extracts were safe since they induced no irritation in the HET-CAM test. In conclusion, environmentally friendly extraction of bioactive components from R. damascena leaves were suggested, with the potential to be exploited as an anti-aging and whitening ingredient for further use in cosmeceutical area.
Keywords: Rosa damascene, Green extraction, Antioxidant, Anti-tyrosinase, Collagen, Elastin; Hyaluronan, Irritation
Funding: The authors are grateful for the research funding provided by the Master of Science Program in Cosmetic Science, Faculty of Pharmacy, Chiang Mai University, Thailand, as well as the support for registration fees and travel expenses from the Graduate School at Chiang Mai University.
Citation: Jittasai, P., Kanjanakawinkul, W., Yawootti, A. and Chaiyana, W. 2023. Phytochemical compositions and cosmeceutical activities of Rosa damascena Mill. leaf extracts from environmentally friendly extraction technique. Natural and Life Sciences Communications. 23(1): e2024008.
INTRODUCTION
The most prominent of all aromatics, medicinal, and aesthetic plants is the Damask rose (Rosa damascena Mill.), a deciduous shrub, which is the most important Rosa species that is grown for rose water and oil, mostly used in the perfume industry and as culinary flavorings (Shabbir et al.,2020). Due to the presence of numerous phytochemical substances such as alkaloids, phenolic acids, flavonoids, and other phenolic compounds, the R. damascena has been proven to have antioxidant, antidiabetic, anti-HIV, antibacterial, anti-inflammatory, and cardiotonic effects (Galal et al., 2022). It is claimed that R. damascena first appeared in ancient Persia (modern-day Iran), from which they later traveled to Europe and Northern Africa. In Western Europe. R. damascena were already grown as garden roses by the 14th century (Rusanov et al., 2009). It has been widely disseminated in China, Saudi Arabia, Spain, France, Morocco, Turkey, India, Bulgaria, Turkey, Syria, and Morocco (Liu et al., 2020).
The R. damascena essential oils is a noticeable cosmetic active ingredient for antioxidants, anti-inflammatory, and antibacterial activity. A significant amount of residue is created during the production of R. damascena essential oil, and it is typically thrown off as industrial waste without being properly utilized. Not only does this cause environmental contamination, but it also wastes a lot of resources (Liu et al., 2020). A number of components, such as blossom remnants, leaves, and stems, are discarded as waste during the manufacturing of R. damascena essential oils (Galal et al., 2022). A significant amount of the R. damascena leaves have been discarded as waste, not only during the harvesting process but also during pruning for flowering. Due to their biologically active substances, such as alkaloids, flavonoids, and phenolic compounds, the pruning wastes of R. damascena could be recycled (Galal et al., 2022). The extraction of active substances from R. damascena leaves would be another way of utilizing the agricultural waste. Since previous research has found that R. damascena leaves contained a number of biological active compounds, such as alkaloids, flavonoids, phenolic compounds, etc. (Galal et al., 2022), it could be used as an active component in cosmetics, such as anti-aging products.
However, the anti-aging and whitening potential of R. damascena leaf extract has not been reported. Therefore, it was interesting to extract the bioactive components from R. damascena leaves, especially by using conventional maceration methods, to be applied as cosmeceutical active for anti-aging and whitening.
MATERIALS AND METHODS
R. damascena leaf materials
R. damascena fresh leaf was obtained as a gift from Chulabhorn Royal Pharmaceutical Manufacturing Facilities by Chulabhorn Royal Academy, Sattahip, Chon Buri during October and November 2022. The plant material was dried in a hot-air oven and ground into a fine powder, which was stored in a tightly closed container and kept in the dark until further use.
Extraction of R. damascena leaves
Conventional maceration
The dried R. damascena leaf powder was macerated in a weight ratio of 1:5 for three cycles of 24 h each in 95% v/v ethanol or 50% v/v ethanol. The residue of R. damascena leaf powder was removed by filtration through the qualitative Whatman grade 1 filter paper. Then, a Buchi rotary evaporator was used to evaporate the solvent. Two independent extractions were performed separately. In preparation for subsequent studies, the solvent extraction extract was stored at 4 °C.
Infusion
The dried R. damascena leaf powder was infused in boiling DI water at a weight ratio of 1:5 for 5 min. The residue of R. damascena leaf powder was removed by filtration through the qualitative Whatman grade 1 filter paper. The filtrate was frozen and dried to remove the water. The infusion extracts were stored at 4 °C pending additional tests.
Digestion
The dried R. damascena leaf powder was infused in warm 95% v/v ethanol at a weight ratio of 1:5 for 5 min. The residue of R. damascena leaf powder was removed by filtration through the qualitative Whatman grade 1 filter paper. The filtrate was frozen and dried to remove the water. The infusion extracts were stored at 4 °C pending additional tests.
Ultrasonic extraction
The dried R. damascena leaf powder was extracted using DI water or 95% v/v ethanol at a weight ratio of 1:5 with the assistance of an ultrasonic bath for 5 min. The residue of R. damascena leaf powder was removed by filtration through the qualitative Whatman grade 1 filter paper. The filtrate was frozen and dried to remove the water. The extract from ultrasonic extraction was stored at 4 °C pending additional tests.
Micellar extraction
The micellar solutions of lauryl glucoside (Plantacare® 1200) were used as the solvent for the micellar extraction at a concentration of 8 mM, which is above its critical micellar concentrations (CMC) of >7 mM (Prokhorova and Glukhareva, 2011). The dried R. damascena leaf powder was extracted using 8 mM of Plantacare® 1200 aqueous solution or ethanolic solution at a weight ratio of 1:5 for 5 min with the assistance of a magnetic stirrer. The residue of R. damascena leaf powder was removed by filtration through the qualitative Whatman grade 1 filter paper. The filtrate was frozen and dried to remove the water. The extract from micellar extraction was stored at 4 °C pending additional tests.
Microwave extraction
The dried R. damascena leaf powder was extracted using DI water at a weight ratio of 1:5 with a microwave oven set at 800 W for 5 min. The residue of R. damascena leaf powder was removed by filtration through the qualitative Whatman grade 1 filter paper. The filtrate was frozen and dried to remove the water. The extract from microwave-assisted extraction was stored at 4 °C pending additional tests.
Pulsed electric fields (PEF) extraction
The dried R. damascena leaf powder was extracted using DI water or 95% v/v ethanol at a weight ratio of 1:5 with the assistance of PEF set at 24 V, 200 W direct current for 5 min, regarding the method of Chaiyana et al. (2020). The residue of R. damascena leaf powder was removed by filtration through the qualitative Whatman grade 1 filter paper. The filtrate was frozen and dried to remove the water. The extract from PEF extraction was stored at 4 °C pending additional tests.
Chemical composition determination of R. damascena leaf extracts
High performance liquid chromatography (HPLC)
The chemical profile of the R. damascena leaf extracts were investigated by HPLC, which is modified from the method of Nouri et al. (2020). Briefly, eluents A and B, which are methanol and 0.1% v/v aqueous formic acid, respectively, was used for the mobile phase. Before the analysis, the mobile phase was filtered through a 0.45 µm nylon filter membrane and degassed in a sonication chamber for 30 min. The following elution condition: 10% A, 0 min; 15% A, 0-5 min; 40% A, 5-15 min; 70% A, 15-25 min; 70% A, 25-28 min;10% A, 28-28.01 min; 10% A, 28.01-35 min. The flow rate was 1 mL/min. The HPLC system with a diode array detector (DAD) set at 280 nm was used to detect the chemical components of the R. damascena leaf extracts. The sample solution, with a volume of 20 µL, was injected into the HPLC system. Various reference standards were used for the HPLC analysis, including kojic acid, chlorogenic acid, epigallocatechin gallate (EGCG), rutin trihydrate, and/or rosmarinic acid. There were two runs of the experiments.
Total phenolic content determination by Folin-Ciocalteu (FC) method
The R. damascena leaf extracts were investigated for their total phenolic contents via the Folin–Ciocalteu method, which was modified by following the method of Chaiyana et al. (2017). Briefly, 100 µL of FC reagent was mixed with 20 µL of 1 mg/mL sample solution. After incubation for 4 min, 80 µL of sodium carbonate solution was added. The resulting combination was allowed to sit for 2 h at room temperature in the dark. A multimode detector was used to measure the mixture's absorbance at 760 nm. For the construction of the standard curve, gallic acid was employed. Gallic acid equivalent (GAE) values, which represent mg of gallic acid/g of the R. damascena leaf extracts, was used to report the results. The experiments were repeated three times.
Total flavonoid content determination by aluminum chloride colorimetric method
The R. damascena leaf extracts were investigated for their total flavonoid contents following the method of Chaiyana et al. (2017). In brief, 80 µL of AlCl3 solution and 100 µL of CH3COOK solution was combined with 20 µL of 1 mg/mL sample solution. The resulting mixture was incubated for 30 min at room temperature and in the dark. A microplate reader was used to measure the mixture's absorbance at 415 nm. Quercetin equivalent (QE) values, which represent the mg of quercetin/g of the R. damascena leaf extracts, was used to report the results. The experiments were repeated three times.
Antioxidation activities determination
2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging assay
The DPPH assay, modified from the techniques of Chaiyana et al. (2017), was used to test the capacity to scavenge DPPH• free radicals of the R. damascena leaf extracts (Chaiyana et al., 2017). The DPPH• solution (180 µL) and 1 mg/mL sample solution (20 µL) was combined to create a final volume of 200 µL, which was incubated for 30 min in the dark. A microplate reader set at 520 nm was used to measure the absorbance of the resulting mixture. The following equation was used to compute the scavenging activities: Inhibition (%) = [(A - B) / A] × 100, where A is the absorbance of the DPPH• solution without the sample solution and B is the absorbance of the DPPH• solution with the sample solution. Ascorbic acid, at the same concentration of the sample solution of 1 mg/mL, was used as a positive control. The experiments were repeated three times.
2,2’-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) assay
The R. damascena leaf extracts were investigated for their radical scavenging activity via the ABTS assay, which is modified from the methods of Chaiyana et al., 2017. In brief, the ABTS free radical (ABTS•+) solution was prepared by combining 2.45 mM of potassium persulfate (K2S2O8) solution with 7 mM of ABTS solution in a 2:3 ratio. The mixture was incubated for 16 h at room temperature in the dark. After that, 20 μL of 1 mg/mL sample solution was mixed with 180 μL of 1:20 diluted ABTS•+ solution and kept at room temperature for 5 min. The microplate reader set at 750 nm was used to measure the absorbance of the resulting mixture. The Trolox equivalent antioxidant capacity (TEAC), presenting the amount of Trolox that is equivalent to 1 g of the R. damascena leaf extracts, were calculated using the standard curve constructed by plotting ABTS•+ scavenging activity versus various concentrations of Trolox. Trolox was used as a standard. The ABTS•+ scavenging activity was expressed as Trolox equivalent antioxidant capacity (TEAC), which was calculated using the following equation: TEAC (mM Trolox/g) = [A - B) + 0.7573] / 0.0145, where A is an absorbance of sample solution with the presence of ABTS•+ solution and B is an absorbance of sample solution without the presence of ABTS•+ solution. Ascorbic acid, at the same concentration of the sample solution of 1 mg/mL, was used as the ABTS assay's positive control. The experiments were repeated three times.
Ferric reducing antioxidant power (FRAP) assay
The R. damascena leaf extracts were investigated for their radical scavenging activity via the FRAP assay, which is modified from the methods of Saeio et al. (2011) and Chaiyana et al. (2017). Freshly prepared FRAP reagent was made up of 0.3 M of acetate buffer pH 3.6, 20 mM of FeCl3, and 10 mM of TPTZ solution in 40 mM HCl in the ratio of 10:1:1. After that, 20 µL of each 1 mg/mL sample solutions was combined with 180 µL of FRAP solution and incubated for 5 min. A microplate reader set at 595 nm was used to measure the absorbance of the resulting mixture. Equivalent concentrations (EC1), which denote the amount of ferric-TPTZ reducing ability equivalent to 1 g of the R. damascena leaf extracts, was used to present the results. Ferrous sulfate (FeSO4) was used as a standard and the ferric ions reducing power was expressed as equivalent capacity (EC1), which represented the amount of FeSO4 equivalents per mg of the sample. EC1 was calculated using the following equation: EC1 (mg FeSO4/g) = [(A - B) +0.0211] / 0.0027, where A is an absorbance of sample solution with the presence of FRAP solution and B is an absorbance of sample solution without the presence of FRAP solution. Ascorbic acid, at the same concentration of the sample solution of 1 mg/mL, was used as the FRAP assay's positive control. The experiments were repeated three times.
Anti-tyrosinase activity determination
The R. damascena leaf extracts were investigated for their anti-tyrosinase activities via spectrophotometric methods, which is modified from the method of Manosroi et al. (2010) and Saeio et al. (2011). In brief, 30 µL of 200 units/mL tyrosinase enzyme in PBS pH 6.5, 60 µL of phosphate buffer PBS pH 6.5, and 10 µL of 1 mg/mL sample solution was combined and allowed to stand for 5 min at room temperature. Then, 100 µL of an L-tyrosine or L-DOPA substrate solution in PBS pH of 6.5 was added. The resulting combination was spent 30 min and incubated at room temperature. The absorbance of the resulting mixture was measured using a multimode detector set at 492 nm. The percentage of tyrosinase inhibition was calculated using the equation: inhibition (%) = (1 - A / B) × 100, where A is the mixture's absorbance with the test solution, and B is the mixture's absorbance without the test solution. Kojic acid, at the same concentration of the sample solution of 1 mg/mL, was used as a positive control in the tyrosinase inhibitory activity determinations. The experiments were repeated three times.
Anti-aging activities determination
Collagenase inhibitory activity determination
The collagenase inhibitory activity of the R. damascena leaf extracts was determined via spectrophotometric methods following the method of Thring et al. (2009) and Laothaweerungsawat et al. (2020). Briefly, 50 mM Tricine buffer pH 7.5 was prepared by mixing 400 mM NaCl and 10 mM CaCl2 solutions. Collagenase was dissolved at a concentration of 5 units/mL, whereas synthetic substrate N-[3-(2-furyl)acryloyl]-Leu-Gly-Pro-Ala (FALGPA) was dissolved at a concentration of 2 mM in the tricine buffer pH 7.5. The resulting mixture was incubated with 1 mg/mL sample solution for 15 min before the addition of 150 μL of the substrate solution to start the reaction. After the substrate is added, the absorbance was immediately measured and continuously for 20 min by using a microplate reader set at 340 nm. The percentage of inhibitory activity against the enzyme was calculated using the following equation: inhibition (%) = (1 – A / B) × 100, where A is the absorbance of the mixture with test solution and B is the absorbance of the mixture without test solution. EGCG, at the same concentration of the sample solution of 1 mg/mL, was used as the positive control in the collagenase inhibitory activity determination. The experiments were repeated three times.
Elastase inhibitory activity determination
The elastase inhibitory activity of the R. damascena leaf extracts was determined via spectrophotometric methods following the method of Laothaweerungsawat et al. (2020). Briefly, 40 µL of 4.5 units/mL elastase was mixed with 10 µL of 1 mg/mL sample solution and incubated for 15 min. After that, 1.6 mM AAAVPN in tris HCI buffer pH 8.0 was added to the mixtures to start the reaction. The absorbance of the mixture was immediately measured and tracked continuously for 20 min by using a microplate reader set at 410 nm. The percentage of inhibitory activity against the enzyme was calculated using the following equation: inhibition (%) = (1-A/B) × 100, where A is the absorbance of the mixture with test solution and B is the absorbance of the mixture without test solution. Oleanolic acid, at the same concentration of the sample solution of 1 mg/mL, was used as the positive control in the elastase inhibitory activity determination. The experiments were repeated three times.
Hyaluronidase inhibitory activity determination
The hyaluronidase inhibitory activity of the R. damascena leaf extracts was determined by measuring N-acetyl-glucosamine, which is the product of the reaction of sodium hyaluronate and hyaluronidase, following the method of Laothaweerungsawat et al. (2020). Briefly, 1 mg/mL samples were incubated with 15 units/mL hyaluronidase, at the temperature of 37 °C for 10 min, in an incubator. After that, 0.03 % w/v hyaluronic acid in phosphate buffer pH 5.35 was added and incubated at 37 °C for 45 min. Then bovine serum albumin, made up from sodium acetate, acetic acid, and bovine serum albumin, was added into the incubated mixture to precipitate the hyaluronic acid. The absorbance of the final mixture was measured by using a microplate reader set at 600 nm. The percentage of inhibitory activity against the enzyme was calculated using the following equation: inhibition (%) = (1-A/B) × 100, where A is the absorbance of the mixture with test solution and B is the absorbance of the mixture without test solution. EGCG, at the same concentration of the sample solution of 1 mg/mL, was used as the positive control in the hyaluronidase inhibitory activity determination. The experiments were repeated three times.
Irritation test of R. damascena leaf extracts
The irritation study of the R. damascena leaf extracts were investigated using hen’s egg-chorioallantoic membrane (HET-CAM) assay, following the method of Chaiyana et al. (2017). All eggs were incubated for 7 days in the hatching chamber kept at 37.5 ± 0.5 °C and humidity of 55 ± 7%. A spinning cutting blade was used to remove the top of the eggshell. The inner membrane was carefully removed using forceps to prevent vascular damage after that. The chorioallantoic membrane (CAM) was exposed to 30 µL of 1 mg/mL sample solutions. Immediately following the exposures, the irritation was observed for 5 min. From these data, an irritation score (IS) was calculated using the following equation: IS = [(301 - t(h)) / 300 × 5] + [(301 - t(l)) / 300 × 7] + [(301 - t(c)) / 300 × 9], where t(h) is the time (s) when the first vascular hemorrhage was detected, t(l) is the time (s) when first vascular lysis was detected, and t(c) is the time (s) when the first vascular coagulation was detected. The following was used to calculate and interpret the irritation score (IS): No irritation is defined as 0.0-0.9, a light irritation is 1.0-4.9, a moderate irritation is 5.0-8.9, and a severe irritation is 9-21. The blood vessel networks were examined once more under a microscope after 60 min to see the long-term irritation. Positive and negative controls for the HET-CAM assay was aqueous solutions of 1% w/v sodium lauryl sulfate and normal saline solutions of 0.9% w/v NaCl, respectively.
RESULTS
R. damascena leaf extracts
The external appearance of R. damascena leaf and the extracts are shown in Figure 1. The color of the extracts was different depending on the extracting solvents. Ethanol, both at 95% v/v and 50% v/v, yielded the extracts with a dark green or dark brown color, whereas DI water yielded the extracts with a pale yellowish color. On the other hand, ethanol produced a semisolid extract, while DI water resulted in a dried powder. The likely explanation for the observed differences in the extracts could be the varying effectiveness of the solvents in partitioning soluble plant metabolites while excluding the insoluble cellular residue, resulting in plant extracts that consist of complex mixtures of metabolites in the form of liquids, semisolids, or dry powders after the solvent has been removed (Flórez et al., 2015).
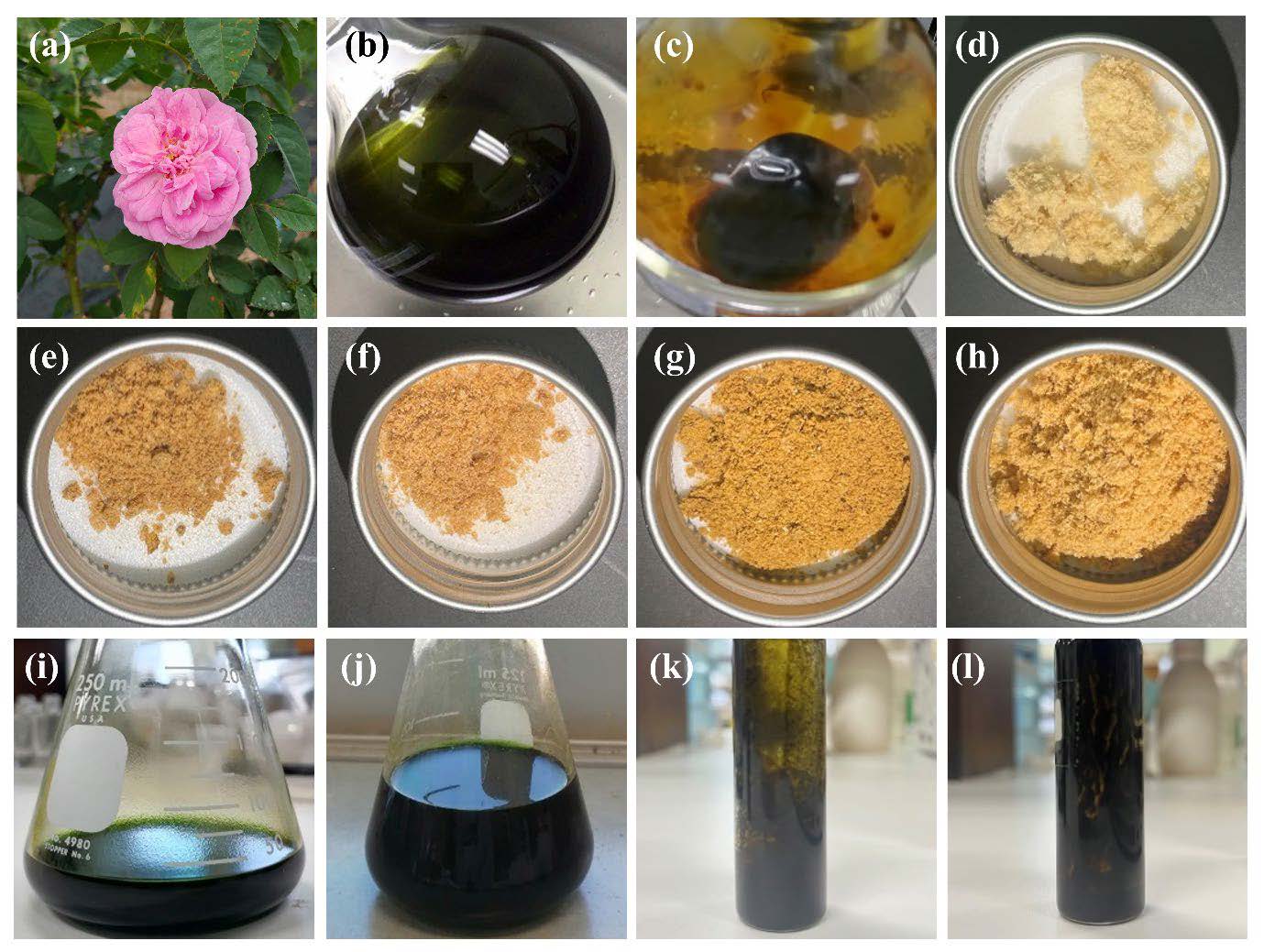
Figure 1. R. damascena flower and leaves (a) and the physical appearance of R. damascena leaf extracts by maceration in 95% v/v ethanol (b), maceration in 50% v/v ethanol (c), infusion using DI water (d), ultrasonic extraction using DI water (e), microwave extraction using DI water (f), micellar extraction using DI water (g), PEF extraction using DI water (h), digestion using 95% v/v ethanol (i), ultrasonic extraction using 95% v/v ethanol (j), micellar extraction using 95% v/v ethanol (k), and PEF extraction using 95% v/v ethanol (l).
The yields of each R. damascena leaf extracts are shown in Figure 2. The results noted that extraction using ethanol yielded a significantly higher extract content than DI water (P < 0.05). However, 50% v/v of ethanol yielded a higher extract content than that of 95% v/v (P < 0.05). The results were in line with the previous study, which reported that L. aromatica leaf extracted with 50% v/v ethanol yielded the significantly highest yield (41.1 ± 1.6% of dry material) compared to that of 75% v/v and 100% v/v of absolute ethanol (P < 0.05) (Do et al., 2014). Besides, the biphasic solvents have been reported to have better performance than monophasic solvents (polar or non-polar solvent alone) on the extraction efficiency (Do et al., 2014). Despite the relatively low content of the extract obtained through DI water extraction, aqueous-based methods exhibit significant promise, mainly due to the cost-effectiveness, safety, and abundant availability of water as a solvent (Handa et al., 2008). Moreover, the reduced reliance on toxic organic solvents aligns with the rising expectations of consumers, growing environmental awareness, and evolving processing standards (Handa et al., 2008).
Aside from the effects of different solvents, different extraction methods also led to differences in the yield of R. damascena leaf extracts. Maceration was found to yield a significantly higher extract content than the green extraction methods (P < 0.05). The likely explanations were due to the longer duration of extraction and several cycles of maceration. Among the green extraction methods, PEF was found to yield a higher extract content than the others, both using 95% v/v and DI water as the solvents. The findings of this study are consistent with a previous study, which highlights that PEF employs a gentle energy treatment to facilitate the specific extraction of intracellular compounds with high purity. This characteristic sets PEF apart from alternative methods such as ultrasonication, microwave-assisted extraction, and high-voltage electrical discharge (Naliyadhara et al., 2022).

Figure 2. Yield of R. damascena leaf extracts from conventional maceration (CON), infusion/ digestion (IF), ultrasonication (UL), microwave (MCW), micellar (MC), and pulse electric field (PEF) extraction. The letter a, b, c, d, e, and f denoted significant different yield among extracts analyzed using one-way ANOVA with post-hoc Tukey test (P < 0.05).
Chemical compositions of R. damascena leaf extracts
The chemical compositions of R. damascena leaf extracts as shown in Table 1 noted that the extract contained a variety of bioactive compounds, including kojic acid, rutin trihydrate, rosmarinic acid, phenolic compounds, and flavonoids. The findings of this study were consistent with a previous report, indicating that R. damascena leaves are significant sources of flavonoids and phenolic compounds (Hessini et al., 2022). Additionally, glycosides such as kaempferol, cyanidin 3,5-diglycoside, and quercetin, as well as gallic acid, terpenes, anthocyanins, pectin, tannins, carotenoids, and vitamins C, A, B, and K, were also identified (Baydar and Baydar, 2013).Kojic acid, which has been used in cosmetics for its excellent whitening effect by inhibition of tyrosinase activity (Cabanes et al., 1994), was only detected in extracts from the maceration method and the green extraction methods facilitated with heat, including infusion in water and digestion in 95% v/v ethanol. On the other hand, rutin and rosmarinic acid were only found in the aqueous extract. Micellar solution and PEF were found to be suitable for rutin extraction, whereas microwave was found to be the most suitable for rosmarinic acid.
Rutin, a common dietary flavonoid with antimicrobial, anticancer, antidiabetic, and anti-inflammatory properties, has been reported to be successfully extracted using a variety of solvents, including ethanol, methanol, acetone, diethyl ether, isopropanol, ethyl acetate, and their mixtures with water (Gullon et al., 2017). The solubility of rutin in water has been reported to be 0.13 g/L, which was lower than that in ethanol (5.5 g/L) (Gullon et al., 2017). The present investigation discovered that micellar solution, which contains a surfactant that acts as a solubilizer, might improve rutin extraction performance. On the other hand, PEF successfully extracted rutin from the R. damascena leaf by using an electric field to cause irreversible poration in the cell membrane and increase membrane permeability (Naliyadhara et al., 2022). In contrast to micellar and PEF extraction which yielded high contents of rutin (88.3% w/w and 87.1% w/w, respectively), rutin was found in lower amount in the extraction from ultrasound (61.3% w/w), microwave (46.4% w/w), and the least in infusion (14.0% w/w). The likely explanation could be due to the heat labile properties of rutin (Gullon et al., 2017).
Rosmarinic acid, a phenolic compound that is an ester of caffeic acid with antiviral, antibacterial, anti-inflammatory, and antioxidant effects (Petersen and Simmonds, 2003), is highly soluble in hydrophilic solvents (Debersac et al., 2001). Therefore, rosmarinic acid was detected only in the extracts using DI water. Microwave assisted extraction, which was based on heating the solvent by application of microwave energy (Flórez el al., 2015), was found to be the most suitable method for the rosmarinic acid extraction. is based on heating the solvent by application of microwave energy.
Additionally, total phenolic and flavonoid content was found to be greater in aqueous extracts than in ethanolic extracts (P < 0.05). The R. damascena leaf extracts from ultrasonic and microwave extraction using DI water contained the significantly highest phenolic content of 356.8 ± 2.6 and 343.5 ± 13.2 mg gallic acid/g extract, respectively (P < 0.05), which was comparable to that from conventional maceration method (351.1 ± 2.5 mg gallic acid/g extract). Comparatively, R. damascena demonstrates potential use due to the higher content of phenolic compounds and flavonoids detected in its leaf extract compared to extracts from other plants. For instance, the leaf extract of L. strychnifolium, obtained through the decoction method, has been reported to contain total phenolic and flavonoid contents of 197.82 ± 5.78 mg gallic acid/g extract and 32.22 ± 1.23 mg quercetin/g extract, respectively (Sato et al., 2019; Thilavech et al., 2023). Similarly, garlic leaf hydroethanolic extract exhibits phenolic and flavonoid contents of 86.11 ± 0.73 mg gallic acid/g extract and 11.09 ± 0.23 mg quercetin/g extract, respectively (Sirisa-ard et al., 2023). On the other hand, Dimocarpus longan leaf hydroethanolic extracts have been reported to be abundant in phenolic and flavonoid compounds, nevertheless, the extraction process requires as long as 6 days (Doungsaard et al., 2023). Beyond the significant bioactive potential exhibited by R. damascena, the current study highlights the potential of using DI water as a green solvent, accompanied by various methods to enhance the extraction efficacy. Therefore, green extraction methods using DI water as a green solvent that consumed a shorter extraction time could be used instead of the conventional maceration method.
Table 1. Chemical compositions of R. damascena leaf extracts.
|
Extracting solvents |
Extraction method |
Chemical compositions |
||||
|
Kojic acid (% w/w) |
Rutin (% w/w) |
Rosmarinic acid (% w/w) |
TPC (mg gallic acid/g) |
TFC (mg quercetin/g) |
||
|
50% EtOH |
Maceration |
32.1 |
0 |
0 |
351.1 ± 2.5a |
88.4 ± 1.5d |
|
95% EtOH |
Maceration |
33.9 |
0 |
0 |
350.1 ± 2.8a |
115.4 ± 4.1a |
|
|
Digestion |
0 |
0 |
0 |
95.5 ± 3.5f |
56.8 ± 4.3e |
|
|
Ultrasound |
0 |
0 |
0 |
245.6 ± 0.12c |
101 ± 0.4c |
|
|
Micellar solution |
0 |
0 |
0 |
134.5 ± 5.2e |
70.3 ± 5.7e |
|
|
PEF |
0 |
0 |
0 |
351.3 ± 2.4a |
62.9 ± 2.9e |
|
Aqueous |
Infusion |
72.4 |
14.0 |
13.5 |
153.4 ± 3.2e |
66.5 ± 0.4e |
|
|
Ultrasound |
0 |
61.3 |
38.7 |
356.8 ± 2.6a |
100.5 ± 4.0b |
|
|
Microwave |
0 |
46.4 |
53.6 |
343.5 ± 13.2a |
103.6 ± 2.8b |
|
|
Micellar solution |
0 |
88.3 |
11.8 |
230.6 ± 1.4d |
90.6 ± 7.5b |
|
|
PEF |
0 |
87.1 |
12.9 |
288.9 ± 2.7b |
85.5 ± 0.9d |
Note: EtOH: ethanol; TPC: total phenolic content; TFC: total phenolic flavonoid content; PEF: pulsed electric fields extraction.
The letter a, b, c, d, e, and f denoted significant different yield among extracts analyzed using one-way ANOVA with post-hoc Tukey test
(P < 0.05).
Cosmeceutical activities of R. damascena leaf extracts
The biological activities related to the cosmetic/cosmeceutical applications of R. damascena leaf extracts, involving DPPH inhibition, ABTS inhibition, ferric reducing antioxidant power, anti-tyrosinase activity on L-tyrosine, anti-tyrosinase activity on L-DOPA, anti-collagenase, anti-elastase, and anti-hyaluronidase activities are shown in Figure 3.

Figure 3. DPPH inhibition (a), ABTS inhibition (b), ferric reducing antioxidant power (c), anti-tyrosinase activity on L-tyrosine (d), anti-tyrosinase activity on L-DOPA (e), anti-collagenase (f), anti-elastase (i), and anti-hyaluronidase activity (j) of R. damascena leaf extracts from conventional maceration (CON), infusion/digestion (IF), ultrasonication (UL), microwave (MCW), micellar (MC), and pulse electric field (PEF) extraction. Positive controls were ascorbic acid (AA), kojic acid (KJ), epigallocatechin gallate (EGCG), or oleanolic acid (OA) depending on the assays. The results represent the mean ± SD of three replicate of each experiment. The letters a, b, c, d, and e denote significant differences among the samples after being analyzed by one-way ANOVA, followed by a Tukey post-hoc test (P < 0.05).
All R. damascena leaf extracts possessed comparable potency for DPPH radical scavenging and ferric reducing antioxidant power. Interestingly, the extracts from micellar and PEF extractions were found to scavenge the ABTS radical, whereas the others exhibited no effect. The ABTS scavenging activities were related to the rutin content of the R. damascena leaf extracts. The results were well consistent with a previous study that observed a strong correlation between rutin content and the antioxidant activities of Florina apple ethanolic extracts (Petkova et al., 2023). Besides, these findings revealed that R. damascena leaf extracts exhibited potent antioxidant properties, as evidenced by their promising performance in DPPH radical scavenging and ferric reducing antioxidant power tests. The results of the study were consistent with the previous research conducted by Baydar and Baydar (2013), confirming the potential use of R. damascena leaf extracts as natural antioxidants in various health products. Furthermore, the most favorable results were obtained from R. damascena leaf extracts using cold methanolic extraction, surpassing the performance of both fresh and spent flowers (Baydar and Baydar, 2013). The observed antioxidant activities within natural extracts are attributed to the collective presence of diverse bioactive compounds, prominently including phenolics and flavonoids (Khelfi et al., 2023; Warinthip et al., 2023; Meziane et al., 2023).
On the other hand, the extracts from maceration using 95% v/v ethanol were found to possess significant anti-tyrosinase activities on both tyrosine and DOPA of 38.1 ± 2.7% and 30.4 ± 3.2%, respectively (P < 0.05), which were well related to the kojic acid content. Since kojic acid has been widely known as a naturally derived compound that functions as a competitive inhibitor of tyrosinase. However, the R. damascena leaf infusion, which contained the highest content of kojic acid exhibited less anti-tyrosinase activities than those of ethanolic extracts. The likely explanation could be due to the other phytochemical component in the infusion, which exhibited superior anti-tyrosinase activities. Previous study has revealed the improved efficiency of tyrosinase inhibitory activity of several kojic acid derivatives (Cardoso rt al., 2021). Although it has been reported that R. damascena exhibited strong anti-tyrosinase activity, with competitive and uncompetitive effects that were 10 times more potent than the positive control kojic acid, those effects were attributed to the essential components of the flower part, as extensively documented in herbal references (Nayebi et al., 2017). Notably, the present study is the first to report the anti-tyrosinase activities of R. damascena leaf extracts, despite the moderate effect.
The anti-collagenase activities demonstrated that the extracts from micellar and PEF extractions were found to be effective and comparable to those obtained through the conventional maceration methods, whereas the others exhibited no effect. These findings emphasize the advantageous characteristics of micellar and PEF extraction, notably in abbreviating the extraction process. Only 5-min duration for micellar and PEF extraction yielded the extracts exhibiting comparable collagenase inhibition potency to those obtained through the conventional maceration method, which extended over a 3-day period. Interestingly, the potency of both micellar and PEF extracts in inhibiting collagenase (69.1 ± 10.1% and 54.8 ± 19.1%, respectively) were equivalent to that of EGCG, a well-known anti-aging compound (76.0 ± 1.2%). However, the analyzed results did not show significant differences, partly due to the wide standard deviation. Conversely, the conventional maceration using 95% and 50% ethanol yielded the extracts with remarkable elastase inhibitory activities, with the inhibition of 52.1 ± 2.9 and 58.0 ± 8.0, respectively. Subsequent PEF extraction, employing DI water, demonstrated a comparatively moderate elastase inhibitory activity of 31.1 ± 5.5%. As the loss of collagen, degeneration in the elastic fiber network, and loss of hydration contribute to dermal atrophy (Uitto, 2008), a strategy aimed at reducing skin wrinkles involves inhibiting collagenase, elastase, and hyaluronidase. Therefore, R. damascena leaf extracts exhibit promising potential for reducing skin wrinkles.
Irritation effects of R. damascena leaf extracts
The observed irritant effects resulting from the exposure to the test compounds are presented in Table 2. The positive control, consisting of a 1% w/v solution of sodium lauryl sulfate, induced vascular hemorrhage, lysis, and coagulation within 5 min of exposure, resulting in an IS of 11.95 ± 0.49, indicating a severe level of irritation. In contrast, the negative control of normal saline solution did not induce any signs of irritation even after a 60-min exposure period. The reliability of the results is supported by the positive outcomes obtained from the positive control and the negative outcomes observed with the negative control. This consistency provides compelling evidence for the validity of the experimental procedure. Besides, the CAM exposed to R. damascena leaf extracts for 5 and 60 min had no irritation sign. Therefore, all R. damascena leaf extracts were considered safe and suggested for further application in cosmetic/cosmeceutical area.
Table 2. Irritation effects of R. damascena leaf extracts in HET-CAM test.

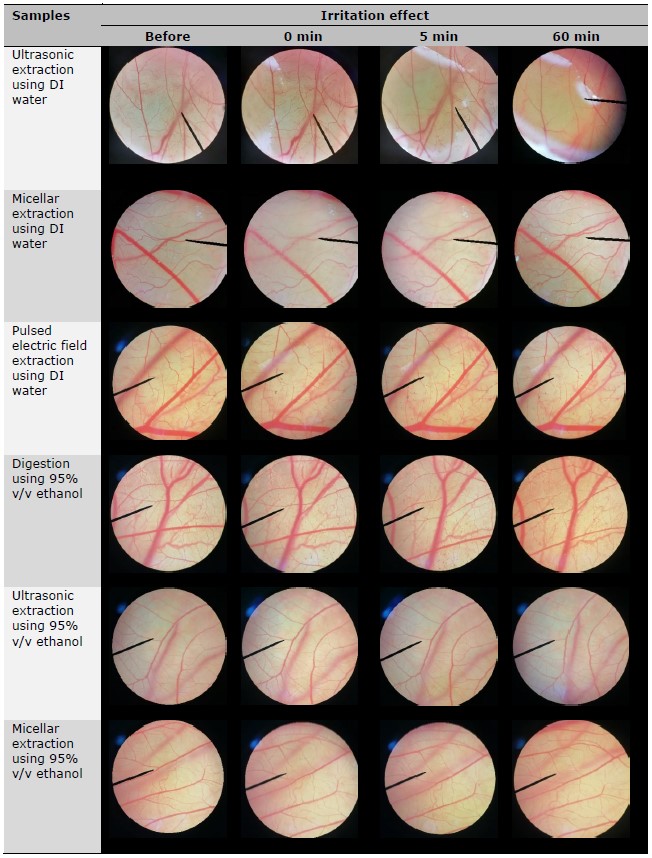
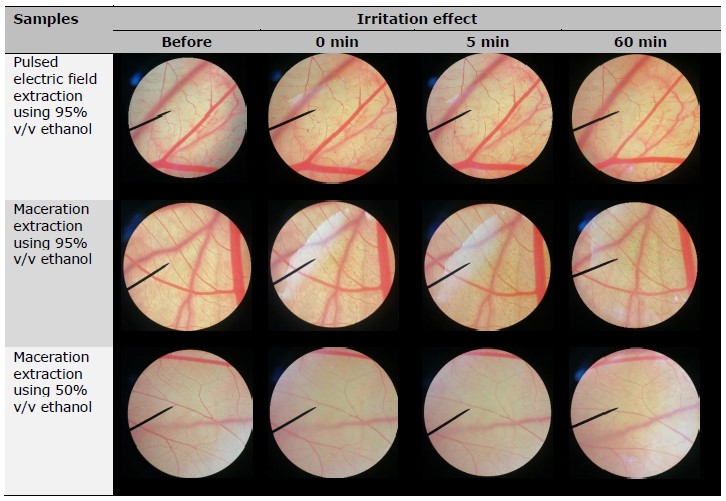
CONCLUSION
The R. damascena leaf was successfully extracted by the green extraction methods using both ethanol and DI water. However, the external appearance of the extract from different solvents was distinct. The ethanolic extracts were a dark green color, whereas the aqueous extracts were clear and pale yellowish. Although the aqueous extracts had lower yields than that of the ethanolic extracts, they contained significant bioactive compounds, including rutin and rosmarinic acid. All R. damascena leaf extracts possessed comparable potency for DPPH radical scavenging and ferric reducing antioxidant power. Interestingly, the extracts from micellar and PEF extractions were found to significantly scavenge the ABTS radical, which were related to their rutin contents. Their collagenase inhibitory activities (69.1 ± 10.1% and 54.8 ± 19.1%, respectively) were equivalent to those of EGCG, a well-known anti-aging compound (76.0 ± 1.2%). On the other hand, the extracts from maceration using 95% v/v ethanol were found to possess significant anti-tyrosinase activities on both tyrosine and DOPA of 38.1 ± 2.7% and 30.4 ± 3.2%, respectively (P < 0.05), which were well related to the kojic acid content. All R. damascena leaf extracts were safe since they induced no irritation in the HET-CAM test. In brief, the environmentally friendly extraction of bioactive components from R. damascena leaves were suggested, with the potential to be exploited as an anti-aging and whitening ingredient for further use in the cosmeceutical and cosmetic area.
ACKNOWLEDGEMENTS
The authors are grateful for the dried materials of Rosa damascena Mill. leaves from Chulabhorn Royal Pharmaceutical Manufacturing Facilities by Chulabhorn Royal Academy. Furthermore, the authors would like to thank the Faculty of Pharmacy, Chiang Mai University, Chulabhorn Royal Pharmaceutical Manufacturing Facilities by Chulabhorn Royal Academy, and Department of Electrical Engineering, Faculty of Engineering, Rajamangala University of Technology Lanna for providing all instruments and facilities.
AUTHOR CONTRIBUTIONS
Pipat Jittasai conducted the experiments, performed the statistical analysis and data visualization, as well as wrote the manuscript. Watchara Kanjanakawinkul, Artit Yawootti, and Wantida Chaiyana assisted in planning the experiment and validated the experimental process during the research. Wantida Chaiyana verified the accuracy of all analysis and data visualization, as well as the manuscript's writing, reviewing, and editing. All authors have read and approved the final manuscript.
CONFLICT OF INTEREST
The authors declare that they hold no competing interests.
REFERENCES
Baydar, N.G. and Baydar, H. 2013. Phenolic compounds, antiradical activity and antioxidant capacity of oil-bearing rose (Rosa damascena Mill.) extracts. Industrial Crops and Products. 41: 375-380.
Cabanes, J., Chazarra, S., and Garcia-Carmona, F. 1994. Kojic acid, a cosmetic skin whitening agent, is a slow-binding inhibitor of catecholase activity of tyrosinase. Journal of Pharmacy and Pharmacology. 46(12): 982-985.
Chaiyana, W., Punyoyai, C., Somwongin, S., Leelapornpisid, P., Ingkaninan, K., Waranuch, N., et al. 2017. Inhibition of 5α-reductase, IL-6 secretion, and oxidation process of Equisetum debile Roxb. ex vaucher extract as functional food and nutraceuticals ingredients. Nutrients. 9(10): 1105.
Chaiyana, W., Sirithunyalug, J., Somwongin, S., Punyoyai, C., Laothaweerungsawat, N., Marsup, P., et al. 2020. Enhancement of the antioxidant, anti-tyrosinase, and anti-hyaluronidase activity of Morus alba L. leaf extract by pulsed electric field extraction. Molecules. 25(9): 2212.
Cardoso, R., Valente, R., Souza da Costa, C.-H., da S., Gonçalves Vianez, Jr J.-L., Santana da Costa, K., de Molfetta, F-A., and Nahum Alves, C. 2021. Analysis of kojic acid derivatives as competitive inhibitors of tyrosinase: A molecular modeling approach. Molecules. 26(10): 2875.
Doungsaard, P., Chansakaow, S., Poomanee, W., Sirithunyalug, J., Intasai, N., and Leelapornpisid, P. 2023. Antioxidant, anti-tyrosinase, antiglycation and safety of longan leaf extract for cosmeceutical application. Natural and Life Sciences Communications. 22(3): e2023052.
Debersac, P., Vernevaut, M.-F., Amiot, M.-J., Suschetet, M., Siess, M.-H. 2001. Effects of a water-soluble extract of rosemary and its purified component rosmarinic acid on xenobiotic-metabolizing enzymes in rat liver. Food and Chemical Toxicology. 39(2): 109-117.
Do, Q.D., Angkawijaya, A.E., Tran-Nguyen, P.L., Huynh, L.H., Soetaredjo, F.E., Ismadji, S., et al.2014. Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. Journal of Food and Drug Analysis. 22(3): 296-302.
Flórez, N., Conde, E., and Domínguez, H. 2015. Microwave assisted water extraction of plant compounds. Journal of Chemical Technology & Biotechnology. 90(4): 590-607.
Galal, T.M., Al-Yasi, H.M., Fawzy, M.A., Abdelkader, T.G., Hamza, R.Z., Eid EM, et al.2022. Evaluation of the phytochemical and pharmacological potential of Taif’s rose (Rosa damascena Mill var. trigintipetala) for possible recycling of pruning wastes. Life. 12(2): 273.
Gullon, B., Lú-Chau, T.A., Moreira, M.T., Lema, J.M., Eibes, G. 2017. Rutin: A review on extraction, identification and purification methods, biological activities and approaches to enhance its bioavailability. Trends in Food Science & Technology. 67: 220-235.
Handa, S.S. 2008. An overview of extraction techniques for medicinal and aromatic plants. Extraction Technologies for Medicinal and Aromatic Plants, 1(1): 21-40.
Hessini, K., Wasli, H., Al-Yasi, H.M., Ali, E.F., Issa, A.A., Hassan, F.A., et al. 2022. Graded moisture deficit effect on secondary metabolites, antioxidant, and inhibitory enzyme activities in leaf extracts of Rosa damascena Mill. var. trigentipetala. Horticulturae. 8(2): 177.
Khelfi, S., Zerizer, S., Foughalia, A., Tebibel, S., Bensouici, C., and Kabouche, Z. 2023. The antioxidant activity and the anti-inflammatory effect of Citrus sinensis L. fruit on intestinal inflammation induced by hyperhomocysteinemia in mice. Natural and Life Sciences Communications. 22(1): e2023009.
Laothaweerungsawat, N., Sirithunyalug, J., and Chaiyana, W. 2020. Chemical compositions and anti-skin-ageing activities of Origanum vulgare L. essential oil from tropical and mediterranean region. Molecules. 25(5): 1101.
Liu, W.-y., Chen, L.-y., Huang, Y.-y., Fu, L., Song, L.-y., Wang, Y.-y., et al. 2020. Antioxidation and active constituents analysis of flower residue of Rosa damascena. Chinese Herbal Medicines. 12(3): 336-341.
Manosroi, A., Jantrawut, P., Akihisa, T., Manosroi, W., and Manosroi, J. 2010. In vitro anti-aging activities of Terminalia chebula gall extract. Pharmaceutical Biology. 48(4): 469-481.
Meziane, Y., Yakoubi, R., Megateli, S., and Chaouia, C. 2023. In vitro assessment of total bioactive contents and antioxidant capacity of grape juices extracts of table and wine varieties from algeria and their correlations. Natural and Life Sciences Communications. 22(3):e2023050.
Naliyadhara, N., Kumar, A., Girisa, S., Daimary, U.D., Hegde, M., and Kunnumakkara, A.B. 2022. Pulsed electric field (PEF): Avant-garde extraction escalation technology in food industry. Trends in Food Science & Technology. 22(2022): 238-255.
Nayebi, N., Khalili, N., Kamalinejad, M., and Emtiazy, M. 2017. A systematic review of the efficacy and safety of Rosa damascena Mill. with an overview on its phytopharmacological properties. Complementary Therapies in Medicine. 34: 129-140.
Nouri, A., Mirabzadeh, M., Safari, N., and Ebadi, M.T. 2020. Evaluation of essential oil composition and rosmarinic acid content in lemon balm (Melissa officinalis L.) cultivated in south of Iran. Journal of Medicinal Plants and By-Products. 9(2): 159-166.
Petersen, M. and Simmonds, M.S. 2003. Rosmarinic acid. Phytochemistry. 62(2): 121-125.
Petkova, N., Bileva, T., Valcheva, E., Dobrevska, G., Grozeva, N., Todorova, M., and Popov, V. 2023. Non-polar fraction constituents, phenolic acids, flavonoids and antioxidant activity in fruits from Florina apple variety grown under different agriculture management. Natural and Life Sciences Communication. 22(1): e2023012.
Prokhorova, G. and Glukhareva, N. 2011. Micellization in aqueous solutions of mixed surfactants containing alkylpolyglucosides. Colloid Journal. 73(6): 841-845.
Rusanov, K., Kovacheva, N., Stefanova, K., Atanassov, A., and Atanassov, I. 2009. Rosa damascene-genetic resources and capacity building for molecular breeding. Biotechnology & Biotechnological Equipment. 23(4): 1436-1439.
Saeio, K., Chaiyana, W., and Okonogi, S. 2011. Antityrosinase and antioxidant activities of essential oils of edible Thai plants. Drug Discoveries & Therapeutics. 5(3): 144-149.
Sato, V.H., Chewchinda, S., Nuamnaichati, N., Mangmool, S., Sungthong, B., Lertsatitthanakorn, P., Ohta, S., and Sato, H. 2019. Pharmacological mechanisms of the water leaves extract of Lysiphyllum strychnifolium for its anti-inflammatory and anti-hyperuricemic actions for gout treatment. Pharmacognosy Magazine. 5(60): 98-106.
Shabbir, F., Hanif, M.A., Ayub, M.A., Jilani, M.I., Rahman, S. 2020. Damask rose. In Medicinal Plants of South Asia. Elsevier, Amsterdam, Netherlands. pp. 217-230.
Sirisa-ard, P., Pholsonklam, K., Satchachai, A., Tragoolpua, Y., and Kaewkod, T. 2023. Antioxidant, antibacterial activities and cytotoxicity of garlic leaf extract from garlic waste. Natural and Life Sciences Communications. 22(4): e2023059.
Thilavech, T., Sutiyaporn, A., Kanchanadumkerng, P., Sato, V.H., Parichatikanond, W., Charoenwiwattanakij, P., and Chewchinda, S. 2023. Development of gummy jelly incorporated with Lysiphyllum strychnifolium leaf extract and its antioxidant and α-glucosidase inhibitory activities. Natural and Life Sciences Communications. 22(2): e2023019.
Thring, T.S., Hili, P., and Naughton, D.P. 2009. Anti-collagenase, anti-elastase and anti-oxidant activities of extracts from 21 plants. BMC Complementary and Alternative Medicine. 9(1): 27.
Uitto, J. 2008. The role of elastin and collagen in cutaneous aging: Intrinsic aging versus photoexposure. Journal of drugs in dermatology: Journal of Drugs in Dermatology. 7(2 Supplement): s12-16.
Warinthip, N., Liawruangrath, B., Natakankitkul, S., Rannurags, N., Pyne, S.G., and Liawruangrath, S. 2023. Chemical constituents antioxidant and antibacterial activities of the leaves and flowers from Gardenia carinata Wallich. Natural and Life Sciences Communications. 22(1): e2023003.
OPEN access freely available online
Natural and Life Sciences Communications
Chiang Mai University, Thailand. https://cmuj.cmu.ac.th
Pipat Jittasai1, Watchara Kanjanakawinkul2, Artit Yawootti3 and Wantida Chaiyana1, *
1 Department of Pharmaceutical Sciences, Faculty of Pharmacy, Chiang Mai University, Chiang Mai 50200, Thailand.
2 Chulabhorn Royal Pharmaceutical Manufacturing Facilities by Chulabhorn Royal Academy, Phlu Ta Luang, Sattahip District, Chon Buri 20180, Thailand.
3 Department of Electrical Engineering, Faculty of Engineering, Rajamangala University of Technology Lanna, Chiang Mai 50300, Thailand.
Corresponding author: Wantida Chaiyana, E-mail: wantida.chaiyana@cmu.ac.th
Total Article Views
Editor: Wipawadee Yooin,
Chiang Mai University, Thailand
Article history:
Received: June 2, 2023;
Revised: December 21, 2023;
Accepted: December 25, 2023;
Online First: January 5, 2024

