
Optimization of HPMC-Based Oral Fast Dissolving Film of Cetirizine Dihydrochloride
Kamchai Saepang, Tasana Pitaksuteepong, Benjaporn Buranrat, and Supavadee Boontha*Published Date : December 21, 2023
DOI : https://doi.org/10.12982/NLSC.2024.007
Journal Issues : Number 1, January-March 2024
Abstract There are challenges involved in improving the convenience of cetirizine dihydrochloride (CTZ) administration for geriatric and pediatric patients with allergic asthma. Due to the ease of swallowing, oral fast-dissolving films (OFDFs) have been identified as a potential solution. This study aimed to prepare hydroxypropyl methylcellulose (HPMC)-based OFDFs of CTZ using the solvent casting method. Film compositions, including the amounts of HPMC and glycerin, were optimized using a face-centered central composite design based on film thickness, folding endurance (FE), and disintegration time (DT). Nine prepared films were found to be transparent and clear in appearance, demonstrating the homogeneity of the films. The weights and thicknesses of all films were in the ranges of 57-109 mg and 22 - 40 µm. The FE of the prepared films was determined to be 42-156, 30-124, and 17-70 times for 3%, 4%, and 5% (w/w) HPMC-based films, respectively. The DT of the films ranged from 99 ± 13 to 478 ± 21 s as their compositions were altered in terms of the HPMC contents and the amount of glycerin. The film fabricated from 3 % (w/w) HPMC and 10% (w/w polymer mass) glycerin (F3) was identified as optimal due to achieving the highest FE and the least DT. Moreover, regression analysis demonstrated a significant effect of the HPMC and glycerin amounts on the film properties. After adding CTZ in F3 (the optimized film), the CTZ content in the films was 0.84 ± 0.14 mg/cm2. The optimized film could be evaluated further for drug release and drug permeation.
Keywords: Cetirizine dihydrochloride, Film former, Folding endurance, HPMC-based film, Plasticizer
Funding: The project was fully supported by the Thailand science research and innovation fund as well as the University of Phayao (Grant number FF66-RIM047).
Citation: Saepang, K., Pitaksuteepong, T., Buranrat, B., and Boontha, S. 2024. Optimization of HPMC-Based oral fast dissolving film of cetirizine dihydrochloride. Natural and Life Sciences Communications. 23(1): e2024007.
INTRODUCTION
Oral fast-dissolving films (OFDFs) are an alternative preparation for drug administration based on their benefits in terms of rapid duration with dosage accuracy and drug therapy efficacy when compared with liquid dosage forms (Phalguna et al., 2019; Rani et al., 2021). Also, OFDFs are more favored and practical for juvenile and elderly patients who have trouble swallowing tablets (Bhowmik et al., 2009; Katewongsa et al., 2017). At the present time, OFDFs resemble prescription (i.e., sildenafil, ondansetron, and risperidone) and over-the-counter (OTC) medications from many therapeutic groups (i.e., benzocaine, simethicone, and diphenhydramine) (Sri rekha et al., 2014; Wasilewska and Winnicka, 2019).
Cetirizine dihydrochloride (CTZ), a powerful second-generation histamine H-1 antagonist, is useful in the management of allergic rhinitis, chronic urticaria, and allergic asthma (Patel et al., 2016). It has no drowsiness or anticholinergic adverse effects, in contrast to many conventional antihistamines. Although CTZ is rapidly absorbed from the gastrointestinal tract following oral administration with peak plasma concentrations achieved in about 1 hour, there are challenges involved with improving the convenience of CTZ administration for patients, particularly geriatric and pediatric patients. OFDFs offer a potential solution to this issue by allowing easy swallowing and releasing the drug into the oral cavity for oral mucosal absorption or intragastric absorption after swallowing, potentially providing a faster onset of action for an enhanced therapeutic effect. As a result, CTZ-loaded OFDFs are also appropriate for patients with acute allergies, such as hay fever, angioedema, and urticarial during travel due to their ease of administration and potential rapid onset of action. Hydrophilic polymers are used to form a film that quickly dissolves on the tongue or buccal fossa, delivering the drug to the primary diffusion through disintegration when contact with the fluid is made (Fatima et al., 2021; Sevinç Özakar and Özakar, 2021). To achieve optimal performance and ensure the safety of the drug when used, ideal OFDFs should possess certain qualities. These qualities include being flexible, easy to administer, easy to handle, both physically and chemically stable, and achieving pharmaceutical equivalence to commercially available oral tablets with a dosage of 5 mg of CTZ per unit dose. Based on these qualities, the CTZ film should exhibit specific quality attributes such as being thin, possessing adequate mechanical strength, showing fast disintegration, and accommodating a 5 mg drug load within the size range of 1-20 cm2. It has been observed that these attributes are influenced by the formulation and process variables employed during the film preparation. For OFDF formulation variables, film formers and plasticizers are crucial factors in determining the characteristics of OFDFs. The film-forming features of hydroxypropyl methylcellulose (HPMC) have been widely studied (Kumar et al., 2013; Patel et al., 2016). Moreover, it has been reported that HPMC significantly improved the drug's BSC Class II, i.e., griseofulvin, dissolving characteristics (Sievens-Figueroa et al., 2012). Additionally, it is widely used as a film former for commercial OFDF prescription and OTC drugs. Plasticizer is a crucial component of oral film formulations and is commonly used in concentrations of 0-20% by weight of the dry polymer. Plasticizer contributes to making films more flexible and less brittle (Jillani et al., 2022). Glycerin is widely used as a plasticizer for commercial OFDF prescription and OTC drugs (Wasilewska and Winnicka, 2019; Rani et al., 2021). This study aimed to prepare HPMC-based OFDFs of CTZ using the solvent casting method. The amount of HPMC and glycerin as critical material attributes of the films was also optimized for OFDFs. The critical process parameters (CPPs), including drying temperature and time, were kept constant for the preparation of all film formulations using the solvent-casting method. Consequently, the effects of different CPPs on the film characteristics were not investigated in this study.
MATERIALS AND METHODS
Materials
HPMC (Methocel F4M, 4000 cps at 2 (%w/w) and 25 °C) was purchased from Namsiang Co., Ltd, Bangkok, Thailand. CTZ was received as a gift from Bangkok Lab and Cosmetics Co, Ltd, Ratchaburi province, Thailand. Glycerin, disodium hydrogen phosphate (Na2HPO4), sodium dihydrogen phosphate (NaH2PO4), sodium azide (NaN3), sodium chloride (NaCl), sodium hydroxide (NaOH), concentrate hydrochloric acid (conc. HCl) were provided from Union Science Co. Ltd. (Chiang Mai, Thailand).
Preparation of HPMC-based films containing either no drug or CTZ
Prior to preparing the CTZ-loaded film, different formulations of a drug-free film were fabricated using the solvent casting method. A face-centered central composite design (FCCD) was performed for the response surface methodology (RSM) and determined the required number of experimental runs. According to FCCD, film-forming solutions consisting of HPMC (3%, 4%, or 5% (w/w)) and glycerin (0%, 5%, and 10 % (w/w) based on the amount of HPMC) were prepared as summarized in Table 1 and thirteen experimental runs of nine film formulations were set up as summarized in Table 2. For preparing the film-forming solution containing the plasticizer, HPMC was dissolved in distilled water (20 ml). Glycerin was then added to the HPMC solution. The final film formulation was adjusted with distilled water to obtain the final weight of 25 g. After polymer solubilization, all film-forming solutions were left under ambient conditions for 48 hours to allow equilibrium and eliminate air bubbles. An amount of 2.5 g of each of the homogeneous, clear, and bubble-free film-forming solutions was cast in a polystyrene Petri dish (60 mm × 15 mm) and subsequently dried under ambient conditions for 24 hours to avoid quick drying and hence film deformation (Ouda et al., 2020). After drying, the films were peeled off from the Petri dish and kept in a sealed polyethylene bag until further characterization in terms of weight, thickness, folding endurance (FE), and disintegration time (DT).
Table 1. Factors in face-centered central composite design (FCCD) for HPMC-based film-forming solutions.
|
Factor |
Level used |
||
|
Low (-1) |
Medium (0) |
High (+1) |
|
|
X1; % (w/w) HPMC |
3 |
4 |
5 |
|
X2; % (w/w) Glycerina |
0 |
5 |
10 |
Note: a based on dry weight of hydroxypropyl methylcellulose (HPMC)
Characterization of HPMC-based films
Film weight
The films (60 mm × 15 mm) were accurately weighed using a 4-digit analytical balance (AAA 250L, Adam, United Kingdom).
Film thickness
The thickness of the films was measured from five locations (the sites of four different points around the film and the middle point) using a Vernier caliper (Cokraft®, Digital caliper, Sweden) (Alhayali et al., 2019).
Folding endurance
The FE of each of the films was determined by repeatedly folding a film (1 cm × 3 cm) at the same place until it broke apart or lost its integrity. The FE value was defined as the number of folds required to break or crack a film (Elshafeey and El-Dahmy, 2021).
In vitro disintegrating time
The DT of the film was determined using a slide frame method (Göbel et al., 2021). A film strip (2 cm × 3 cm) was fixed in a slide frame. Deionized water (200 µl) was dropped onto the film. The time required for the water to pass through the film was recorded and considered as the DT for the film. Based on the entire unstimulated saliva flow rate, the result is roughly 0.3–0.4 ml/min, and 200 µl of deionized water represents the volume of saliva per min in the mouth (Iorgulescu, 2009).
Optimization study
The optimal formulation was determined using the RSM-based FCCD. The response surface behavior was investigated for the response function (Y) employing the polynomial equation:
Y = β0+β1X1+β2X2+β11X12+β22X22+β12X1X2 (1)
where Y is the predicted response; β0 is constant; β1, β2 β11, β22, and β12 are the regression coefficients for the independent variables; X1 and X2 are the coded levels of the corresponding independent variables.
The FCCD experimental data obtained from the evaluations of all the film formulations (thirteen experimental runs of nine film formulations) were analyzed using Design-Expert® program version 13 (Stat-Ease, Inc, USA) to assess the best-fitted models (i.e., linear, 2FI, quadratic and cubic models). Subsequently, analysis of variance (ANOVA) was performed to determine the significant influence of independent variables in the best-fitted model. All significant independent variables effect (P -value < 0.05) were included the best-fitted model.
Preparation and characterization of CTZ-loaded films
Following the determination of the physical properties, the drug-free film formulations with suitable FE and DT for OFDFs were selected and subjected to the incorporation of CTZ. CTZ (40 mg) was added to the selected film formulations by dissolving CTZ in the optimal film-forming solution (4 g). Afterward, the film-forming solutions containing CTZ were subsequently cast into a Petri dish following the same process of the drug-free films. The physical appearance, weight, thickness, FE, and DT of the prepared CTZ-loaded films were evaluated as described above.
Drug content
The drug content of the films was examined by dissolving each of the prepared films in a beaker prefilled with 10 ml of distilled water (pH 6.8). After the films were completely dissolved, the obtained solution was filtered using a 0.22 µm nylon syringe membrane filter and then analyzed by using the High-Performance Liquid Chromatography (HPLC) technique. The entire process was performed in triplicate to obtain the mean drug content (El-Said et al., 2021).
HPLC analysis
HPLC analysis of CTZ was carried out according to a previously described method (Souri et al., 2011). A Shimadzu HPLC system, consisting of an isocratic pump, an autosampler, and a variable UV–vis detector (LC 2050C, Shimadzu, Japan), was employed. A symmetry C18 column (Hypersil BDS: 4.6 mm ×150 mm, 5 µm particle size) was used as the stationary phase. The mobile phase was a mixture of 50 mM KH2PO4 and acetonitrile (60:40 v/v, pH = 3.5). The flow rate was 1 ml/min. The injection volume was 20 µl. The wavelength for UV detection was 230 nm. System suitability parameters of the HPLC method were assessed prior to using the method in the experiment.
FT-IR analysis
Fourier-Transform Infrared Spectroscopy (FT-IR) analysis was performed on pure CTZ, HPMC, 1:3 weight ratio of CTZ-HPMC physical mixture, blank film, and CTZ-loaded film using an FT-IR spectrophotometer (IRAffinity-1S, Shimadzu Co., Kyoto, Japan). Prior to sample measurement, a spectrum of atmospheric composition as a background was collected and applied for background correction. The samples were placed on a clean crystal of the attenuated total reflection (ATR) apparatus and then separately scanned in the following experimental conditions: a spectral range of 4,000-700 cm-1; a resolution of 4 cm−1 with 45 scans.
Data analysis
Data were statistically analyzed using Sigma Stat software version 3.5 (Systat Software Inc., San Jose, CA, USA). An independent t-test was used to determine the differences between two-group data. In case of more than two-group data, one-way ANOVA with post hoc LSD test was performed. A P-value of less than 0.05 was considered a statistically significant difference.
RESULTS
Preformulation of drug-free films
Prior to drug loading, the optimal HPMC-based films were identified using FCCD. As summarized in Table 2, nine film formulations obtained from thirteen experimental runs were fabricated using different amounts of HPMC (3%, 4%, and 5% (w/w)) and glycerin (0%, 5%, and 10% (w/w)) and then evaluated for their appearance, weight, thickness, FE and DT.
Table 2 Formulation of films generated from face-centered central composite design (FCCD).
|
Experimental run order |
Formulation (F) |
FCCD coding (X1, X2) |
HPMC (% w/w) |
Glycerin (% w/w)a |
Glycerin (g) |
|
1 |
5 |
(0, 0) |
4 |
5 |
0.0500 |
|
2 |
5 |
(0, 0) |
4 |
5 |
0.0500 |
|
3 |
3 |
(-1, +1) |
3 |
10 |
0.0750 |
|
4 |
9 |
(+1, +1) |
5 |
10 |
0.1250 |
|
5 |
7 |
(+1, -1) |
5 |
0 |
0.0000 |
|
6 |
5 |
(0, 0) |
4 |
5 |
0.0500 |
|
7 |
5 |
(0, 0) |
4 |
5 |
0.0500 |
|
8 |
8 |
(+1, 0) |
5 |
5 |
0.0625 |
|
9 |
6 |
(0, +1) |
4 |
10 |
0.1000 |
|
10 |
4 |
(0, -1) |
4 |
0 |
0.0000 |
|
11 |
1 |
(-1, -1) |
3 |
0 |
0.0000 |
|
12 |
2 |
(-1, 0) |
3 |
5 |
0.0375 |
|
13 |
5 |
(0, 0) |
4 |
5 |
0.0500 |
|
Note: FCCD: face-centered central composite design, HPMC: hydroxypropyl methylcellulose. a based on dry weight of HPMC |
|||||
The visual inspection demonstrated that all films (F1-F9) were transparent and smooth (Figure 1), which was similar to the appearance of the HPMC-based film reported by Phalguna et al. (2019).

Figure 1. Appearance of films prepared using various concentrations of hydroxypropyl methylcellulose (HPMC) and glycerin: (a) F1, (b) F2, (c) F3, (d) F4, (e) F5, (f) F6, (g) F7, (h) F8 and (i) F9.
Characteristics of prepared HPMC-based films
Weight: The weight of the films ranged from 57 to 109 mg. An increase in film weight can be attributed to the increased HPMC content in the film casting solution. The amount of glycerin in the film has an insignificant effect on the film weight. The average weight of all the films is given in Table 3.
Table 3. Characteristics of the prepared hydroxypropyl methylcellulose (HPMC) films.
|
Run |
HPMC (% w/w) |
Glycerin (% HPMC mass) |
Thickness (µm) |
Dry weight (mg)a |
FE (times) |
DT (s) |
|
|
1(F1) |
3 |
0 |
22 ± 1 |
57 ± 3 |
42 ± 5 |
99 ± 13 |
|
|
2(F7) |
5 |
0 |
38 ± 2 |
96 ± 1 |
17 ± 1 |
478 ± 21 |
|
|
3(F3) |
3 |
10 |
24 ± 2 |
61 ± 4 |
156 ± 8 |
113 ± 48 |
|
|
4(F9) |
5 |
10 |
40 ± 2 |
109 ± 2 |
70 ± 9 |
363 ± 87 |
|
|
5(F2) |
3 |
5 |
25 ± 4 |
58 ± 8 |
71 ± 20 |
114 ± 20 |
|
|
6(F8) |
5 |
5 |
37 ± 3 |
101 ± 2 |
44 ± 9 |
252 ± 67 |
|
|
7(F4) |
4 |
0 |
30 ± 2 |
75 ± 2 |
30 ± 8 |
174 ± 36 |
|
|
8(F6) |
4 |
10 |
32 ± 3 |
85 ± 2 |
124 ± 8 |
203 ± 16 |
|
|
9(F5) |
4 |
5 |
31 ± 2 |
80 ± 3 |
86 ± 6 |
185 ± 26 |
|
|
10 |
4 |
5 |
29 ± 1 |
81 ± 2 |
75 ± 12 |
160 ± 21 |
|
|
11 |
4 |
5 |
30 ± 3 |
79 ± 1 |
83 ± 7 |
188 ± 61 |
|
|
12 |
4 |
5 |
30 ± 3 |
78 ± 4 |
80 ± 22 |
241 ± 97 |
|
|
13 |
4 |
5 |
29 ± 1 |
77 ± 3 |
83 ± 32 |
218 ± 42 |
|
|
Note: HPMC: hydroxypropyl methylcellulose, FE: folding endurance, DT: disintegration time, a dry weight per 19.64 cm2 film
|
|||||||
Thickness: The thickness of all film formulations was between 22 to 40 µm (Table 3); this result did not exceed the typical range of 5-200 µm for OFDFs. Exceeding the typical range could bring discomfort to the patient (Lai et al., 2018).
FE: The FE of the HPMC-based films prepared was determined to be 42-156, 30-124, and 17-70 times for 3% (F1-3), 4% (F4-F6), and 5% (F7-F9) (w/w), respectively, as presented in Table 3. The FE of the prepared films was increased by increasing the glycerin.
DT: DT is a desirable characteristic of films that dissolve in less than 60 s. The DT of all film formulations ranged from 99 ± 13 to 478 ± 21 s as their compositions were altered in terms of the HPMC contents and the amount of GCR as summarized in Table 3.
According to the characteristics of film data, the RSM plots were generated as shown in Figure 2. The RSM plots showed that the quality attributes of film, including weight (Figure 2a), thickness (Figure 2b), FE (Figure 2c), and DT (Figure 2d) as a function of HPMC and glycerin concentrations. To select the optimal film formulation comprising HPMC and glycerin, data were fitted to the statistical model as summarized in Table 4. The best-fitted models were a two-factorial interaction (2FI) model for weight (R2 =0.9954), a linear model for thickness (R2 = 0.9651), a 2FI model for FE (R2 =0.9334), and a linear model for DT (R2 = 0.7479). Through ANOVA analysis of the best-fitted models, significant variables (P-value < 0.05) were incorporated in the equations with the positive sign indicates the increase in the response due to the corresponding factor.
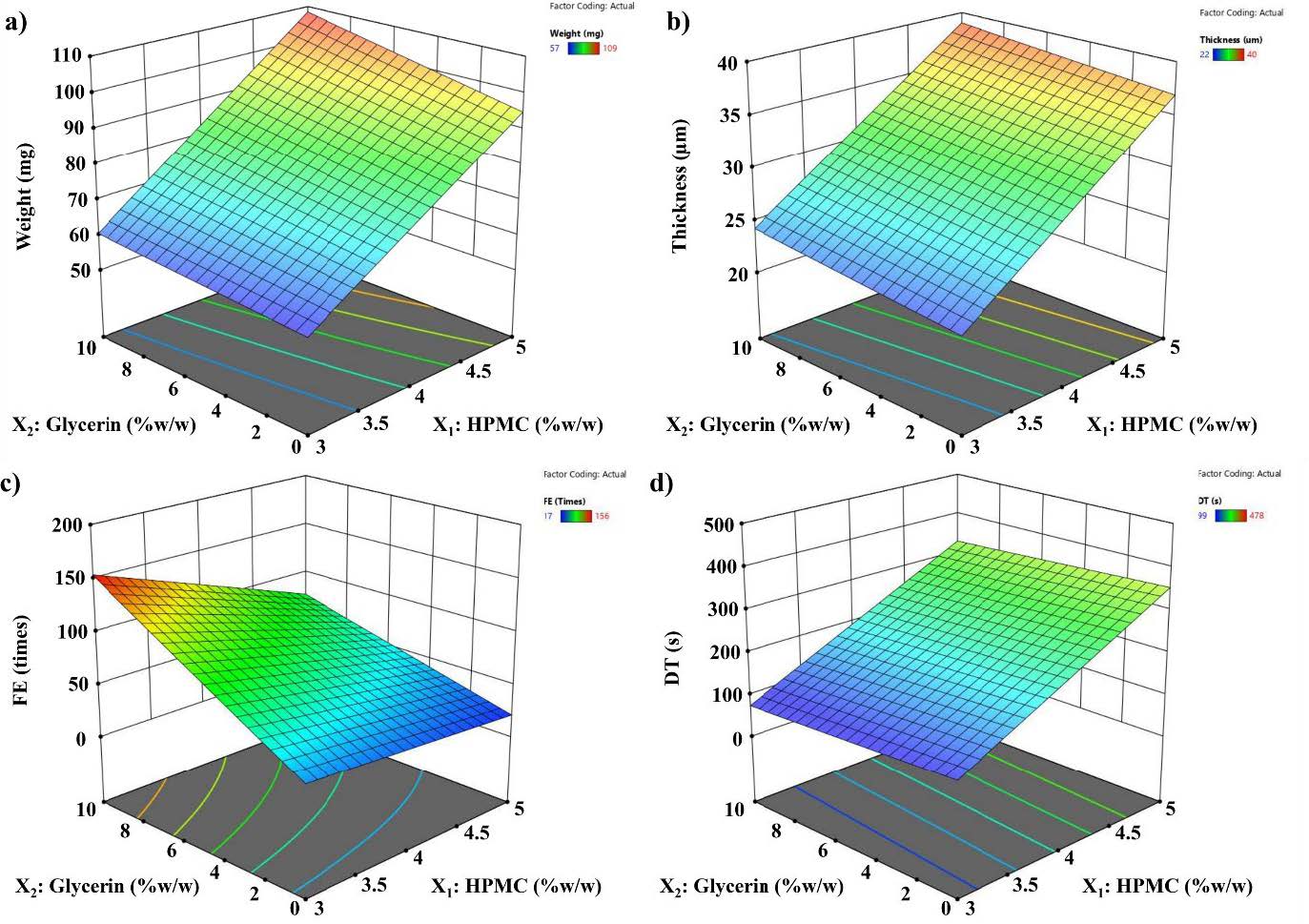
Figure 2. Surface response plots: (a) film weight, (b) film thickness, (c) film folding endurance (FE), and (d) film disintegration time (DT) determined by the slide frame method.
From the plots, it was noted that a lower polymer concentration was required to obtain low DT (20–30 s), while the plasticizer effect was trivial. However, at higher polymer concentrations, higher plasticizer concentration was preferred for lower DT (Figure 2d). A similar result was observed for HPMC-based films with glycerin incorporation greater than 1.5 % w/w as reported by Irfan et al. (2016). Such results can be explained by the effect of the plasticizer on polymer interaction and the higher hydrophilicity of the film. Varying polymer and plasticizer concentrations did not produce films outside the targeted range for content uniformity and/or thickness uniformity. The goal of optimization was to find the best film compositions for producing HPMC-based oral film with a minimum diameter. Therefore, according to the final RSM equation (Table 4) and the preliminary tests, the HPMC and glycerin concentrations were set within the range studied (Table 2) while the responses were fixed at the minimum for thickness and DT and at the maximum for FE. Based on the results obtained from the response surface plot, optimal formulation parameters within the independent variables that reveal the ranges that could result in optimal responses (adequate FE of close to 300 times and low DT of fewer than 60 s) (Yir-Erong et al., 2019; Rani et al., 2021). F3 provided desired responses with the highest FE and lower DT when compared with all prepared film formulations. Thus, F3 was selected to prepare the CTZ-loaded film.
Table 4. Regression analysis for four responses of films.
|
Response |
Model |
R2 |
Predicted R2 |
Adjusted R2 |
Std Dev |
Regression equationa |
|
Weight |
2FI |
0.9954 |
0.9873 |
0.9935 |
1.39 |
79.77 + 21.67X1 + 4.50X2 + 2.25X1X2 |
|
Thickness |
Linear |
0.9651 |
0.9383 |
0.9564 |
1.22 |
30.54 + 7.33X1 |
|
FE |
2FI |
0.9334 |
0.8398 |
0.9049 |
12.55 |
73.92 - 23X1 + 43.50X2 – 15.25 X1X2 |
|
DT |
Linear |
0.7479 |
0.3979 |
0.6848 |
64.56 |
214.46 + 127.83X1 |
|
Note: 2FI: two-factorial interactions, FE: folding endurance, and DT: disintegration time. a X1 and X2 are concentrations of HPMC and glycerin, respectively. |
||||||
Characteristics of CTZ-loaded films and drug contents
CTZ was added to a film to obtain a 1 mg/cm2 sample. The appearance of the CTZ-loaded film is shown in Figure 3. The weight and thickness of the CTZ-loaded film were 66 ±18 mg and 32 ± 4 µm. The FE and DT of the CTZ-loaded film were 58 ± 17 times and 53 ± 22 s. The CTZ-loaded film was heavier and thicker than the blank film (Figures 4a and 4b). However, the CTZ-loaded film showed less FE and shorter DT than the blank film (Figures 4c and 4d). This was due to CTZ being very soluble, resulting in a more soluble film obtained after adding CTZ (Katewongsa et al., 2017). The drug content of the CTZ-loaded film was determined. Using HPLC analysis, the chromatogram peak of the CTZ-loaded film was observed at 3.9 min. The concentration range of 1–20 µg/ml of CTZ was used as the working standard curve. The results showed that the CTZ content in the OFDF films was 0.84 ± 0.14 mg/cm2. Drug recovery in the OFDF films was 84±14 %.
Figure 3. The appearance of hydroxypropyl methylcellulose (HPMC) films (F3): (a) cetirizine (CTZ)-loaded HPMC film as compared with (b) blank HPMC film.

Figure 4. Physical properties of blank and cetirizine (CTZ)-loaded HPMC film based on F3: weight (a), film thickness (b), folding endurance (FE) (c), and disintegration time (DT) (d). The data represent the mean ± SD (n ≥ 3). Asterisk symbols (*) indicate statistically significant differences as compared to the blank film (P < 0.05).
FT-IR analysis
To confirm the absence of any possible chemical interactions between the drug (CTZ) and film matrix (HPMC), a drug-excipient compatibility study was conducted using FT-IR analysis. Figure 5 shows the spectra of the samples studied. Pure CTZ showed the characteristic FT-IR band of the carbonyl (C=O) group at 1736 cm-1 (Figure 5a), which is in agreement with the carboxyl group of CTZ at 1737 to 1739 cm−1 reported in previous studies (Paczkowska et al., 2018; Kumar et al., 2019). The C=O band of the pure drug was not found in the spectra of the HPMC (Figure 5b) and the blank film (Figure 5d). However, it was found in the spectra of the physical mixture of CTZ and HPMC (at a weight ratio of 1:3) (Figure 5c) and the CTZ-loaded film (Figure 5e), indicating that CTZ was in a free state in the prepared film. As compared with the pure drug and the physical mixture, only a minor change in intensity of the C=O band in the CTZ-loaded film was observed. This might be due to the hydrogen bonding between the drug and polymer as previously reported by Pamlényi et al. (2021). According to the previous report, the carboxyl group of CTZ can form hydrogen bonds with the hydroxyl group of HPMC resulting in the decreased peak intensity of the drug in the polymer film (Pamlényi et al., 2021). Based on the FT-IR result, it suggests that there is a lack of interaction between CTZ and HPMC in the CTZ-loaded film prepared in the present study, in which CTZ remains free and compatible with HPMC after film preparation.

Figure 5. Fourier transformed infrared (FT-IR) spectra: pure CTZ (a), HPMC (b), a physical mixture at 1:3 weight ratio of CTZ and HPMC (c), blank film (d) and CTZ-loaded film (e).
DISCUSSION
This study developed and optimized oral dissolving films using HPMC and glycerin as a film-forming polymer and plasticizer, respectively. Based on the scientific literature, HPMC was selected as the film-forming polymer due to its hydrophilic and non-ionic nature, which facilitates rapid hydration of the prepared films upon contact with saliva, resulting in quick dissolution and compatibility with drugs (Dahmash et al., 2021). Glycerin was used as a plasticizer due to its small molecular weight, allowing it to easily penetrate and insert between the polymer chains, thereby significantly impacting the mechanical properties of the films (Bourtoom et al., 2008). Central composite, Box–Behnken, and full factorial designs are frequently used for drug formulation development and optimization. Among these designs, the central composite design requires a smaller number of experiments but it can provide information exclusively on the effect of experiment variables and no need for a three-level factorial experiment for building a second-order quadratic model (Beg et al., 2021, Bhattacharya et al., 2021, Rakić et al., 2014). Therefore, the FCCD was used in the present study to systematically characterize and optimize the CTZ-loaded film based on the HPMC and glycerin concentrations. Nine blank HPMC film formulations were prepared over 13 experimental runs and then evaluated for their appearance, weight, thickness, FE, and DT. To confirm the consistency of prepared films and the amount of the drug in the film, the determination of film thickness and film weight was conducted. The FE test has been suggested to relate to not only determining the strength but also the elongation during the tensile test, which is more suitable for clarifying the actual strength during the manufacturing and dosing processes (Takeuchi et al., 2020). Accordingly, only FE was evaluated for the films prepared in the present study. During the formulation screening for optimization, if the formulation demonstrates favorable FE properties, it is likely that the tensile strength will also be good. The performance of oral films appeared to depend strongly on the film thickness. Takeuchi et al. (2018) studied the effect of film thickness ranging from 10 to 80 µm on DT and reported that the DT of the films increased as the film thickness increased. Our DT result is in good agreement with the previous study, which reported that increasing the concentration of polymer (HPMC) leads to a longer DT of the films. Accordingly, keeping the thickness of the CTZ-loaded films as thin as possible will allow the films to disintegrate and release the incorporated CTZ quickly, facilitating the fast onset of action when used. The RSM data confirmed that the amount of HPMC is a major factor in film weight, film thickness, and DT, whereas the amount of glycerin is a major factor on the FE of the obtained films. The best-fitted models were a 2FI model for weight and FE, and a linear model for thickness and DT. ANOVA analysis of best-fitted model suggested that both the weight and FE of the film were significantly influenced by HPMC, glycerin, and their interaction, whereas HPMC alone significantly impacted on thickness and DT of the resulting film. The positive and negative signs indicate the increase and decrease in the response due to the corresponding factor, respectively as shown in Table 4.
The RSM data of film thickness are represented in Figure 2b. It explains that the films produced from 3% w/w HPMC (F1-F3) were thinner than those produced from 4 %w/w (F4-F6) and 5 %w/w HPMC (F7-F9). At each concentration of HPMC, the thickness of the film slightly increased as a function of glycerin concentration. Based on the regression analysis, the equation suggested a greater effect of HPMC concentration on the film thickness compared with glycerin concentration, supporting that the thickness of the films is directly related to the HPMC concentration in the film formulation. The effect of HPMC concentration on film thickness can be attributed to a higher solid content of polymer in the film (Rani et al., 2021). As shown in Figure 2c, the FE followed a 2FI model. Amongst the two constituent films, HPMC negatively impacted the flexibility of the films, whereas glycerin enhanced the flexibility of the films. Additionally, the interaction between HPMC and glycerin was observed for its positive effect on FE. In general, the tensile strength of dried films depends upon the polymer mass, film thickness, and concentration of the plasticizer. The results indicate that HPMC and glycerin as independent-variable factors impact the film quality, which is in agreement with previous studies (Baniya et al., 2020). It was clearly observed that the FE was affected by the concentration of the plasticizer. It is assumed that glycerin decreases the internal hydrogen bonding between polymer chains, therefore triggering polymer chain relaxation in turn, resulting in the flexibility of polymer films (Irfan et al., 2016). The response surface plots of the DT are represented in Figure 2d. It was observed that the DT generally increased with HPMC concentration but decreased with glycerin concentration. In order to find optimum formulations, the simultaneous optimization of all responses was carried out. Values of thickness (˂ 200 μm) and DT (≤ 60 s) and FE (> 300 times) are ideally preferred for film optimization. Based on the obtained results in this study, all film formulations had an acceptable thickness (22-40 μm); so, DT and FE were considered for optimization. However, it is important to note that the model of RSM optimization generally provides a good fit for the range of independent variables used to establish the model. The actual experiment did not reach the desired DT (≤ 60 s) and FE (> 300 times) and is then unable to create design space for optimization within these limits. Consequently, the optimized formulation was selected by setting the criteria of film quality attributes to a minimum for DT and to a maximum for the FE. The Design-Expert® program suggested four film formulations consisting of HPMC 3.0% (w/w) and glycerin 3.13-10% (w/w). Among these formulations, HPMC 3 % (w/w) and glycerin 10% (w/w polymer mass) (F3) had the least DT (113 ± 48 s) and the highest FE (156 ± 8 times). Additionally, the marketed OFDFs hold a ≤30 s DT with sufficient tensile strength (19–21 N/mm2), FE (≥6), and % endurance (8–10%) (Wasilewska and Winnicka, 2019; Rani et al., 2021). Thus, the film fabricated from HPMC 3 % (w/w) and glycerin 10% (w/w polymer mass) (F3) was identified as the optimal film formulation for preparation of CTZ-loaded films according to achieving the highest FE and the lowest DT based on the data obtained in the present study. The FE of the prepared CTZ-loaded film was approximately 60 times and the DT of the prepared CTZ-loaded film was less than 60 s. Currently, there are no official guidelines available for determining the disintegration time of oral dissolving films. However, published studies have often applied the disintegration time requirements specified for orodispersible tablets, such as within 30 s required by the FDA or 180 s by the European Pharmacopoeia, to oral dissolving film (U.S. Food and Drug Administration (FDA), 2018; European Pharmacopoeia, 2016). In our study, the DT-optimized CTZ-loaded film (53 ± 22 s) met the criteria set by the European Pharmacopoeia, indicating its potential for achieving good compliance in clinical applications. Also, the DT result indicated that the prepared CTZ-loaded films met the criteria for OFDFs as previously described (Baniya et al. 2020; Rani et al., 2021). Using the HPLC technique, the results showed that CTZ content in the film was 0.84 ± 0.14 mg/cm2. Thus, six CTZ-loaded film were required for a 5 mg (dose = 5-10 mg/day). However, CTZ recovery in the film was approximately 84%. This might be due to the variation in film weight and film thickness of CTZ-loaded film. More randomly prepared films should be tested in future drug content studies. Also, a minor interaction between CTZ and HPMC occurred as shown in Figure. 5. Thus, CTZ-loaded film lie out of the range of 85–115% of the label criteria of the United States Pharmacopeia (USP 41). The empirical results reported in this study demonstrate that the optimized CTZ-loaded film with an area of 5.95 cm2 (≈6 cm2) might have the potential to serve as an alternative oral dosage form for a 5 mg CTZ tablet. However, in order to establish the film's feasibility, it is essential to conduct comparative studies against a commercially available CTZ tablet. These studies should encompass various aspects, including dissolution, caco-2 cell-based permeability, and potentially in vivo permeation.
CONCLUSION
The prepared OFDFs have an advantage over the traditional CTZ formulation in terms of drug administration accuracy and adaptability. In this study, the preparation of CTZ-loaded film formulations was achieved. An optimization approach through the design of experiments was adopted to optimize formulation parameters in the preparation of CTZ-loaded film with desirable properties. The weight, thickness, and DT of films increased with increases in the amount of HPMC, whereas the FE of the prepared film decreased with increases in the amount of HPMC. To determine the effect of glycerin, the weight, thickness, FE, and DT of films were increased with increases in the amount of glycerin. However, an increase in the amount of glycerin did not significantly impact the weight, thickness, and DT of the film. While the thicknesses of all the prepared blank films meet the criteria of OFDFs, the FE and DT of all the prepared blank films do not meet the criteria of OFDFs. The optimal film formulation was achieved using 3% w/w HPMC as the film former, with a minimum of 10% glycerin (w/w on a polymer mass) as a plasticizer. The CTZ content in the film was 0.84 ± 0.14 mg/ cm2. The CTZ-loaded film lies out of the acceptance range of the label criteria of the USP. However, the optimized film could be evaluated further for drug release and drug permeation.
ACKNOWLEDGEMENTS
The authors thank the School of Pharmaceutical Sciences, University of Phayao for providing instruments.
AUTHOR CONTRIBUTIONS
Supavadee Boontha designed the study and the experiments, conducted the project, and prepared the manuscript. Kamchai Saepang was responsible for film formulation study and wrote the manuscript. Benjaporn Buranrat and Tasana Pitaksuteepong assisted in conducting the experiments. All authors have read and approved of the final manuscript.
CONFLICT OF INTEREST
The authors declare that they hold no competing interests.
REFERENCES
Alhayali, A., Vuddanda, P.R., and Velaga, S. 2019. Silodosin oral films: Development, physico-mechanical properties and in vitro dissolution studies in simulated saliva. Journal of Drug Delivery Science and Technology. 53: 101122.
Baniya D.P., Pandey, G., Bajaracharya, M., and Dhungana, B.R. 2020. Formulation and evaluation of fast dissolving oral films of cetirizine hydrochloride. Europasian Journal of Medical Sciences. 2: 23-29.
Beg, S. and Rahman, Z. 2021. Central composite designs and their applications in pharmaceutical product development. p.63-76. In Beg, S. (ed) Design of Experiments for Pharmaceutical Product Development. Springer, Singapore.
Bhattacharya, S. 2021. Central composite design for response surface methodology and its application in pharmacy. p.1-19. In Kayaroganam, P. (ed) Response Surface Methodology in Engineering Science. IntechOpen. London.
Bhowmik, D., Krishnakanth, C.B., and Chandira, R.M. 2009. Fast dissolving tablet: An overview. Journal of Chemical and Pharmaceutical Research. 1(1): 163-177.
Bourtoom T. 2008. Plasticizer effect on the properties of biodegradable blend film from rice starch-chitosan. Songklanakarin Journal of Science and Technology. 30(S1):149–165.
Dahmash, E.Z., Iyire, A., and Alyami, H.S. 2021. Development of orally dissolving films for pediatric-centric administration of anti-epileptic drug topiramate - A design of experiments (DoE) study. Saudi Pharmaceutical Journal. 29(7): 635–647.
El-Said, I.A., Aboelwafa, A.A., and ElGazayerly, O.N. 2021. Optimization of taste-masked dapoxetine oral thin films using factorial design: In vitro and in vivo evaluation. Pharmaceutical Development and Technology. 26(5): 522–538.
Elshafeey, A.H., and El-Dahmy, R.M. 2021. Formulation and development of oral fast-dissolving films loaded with nanosuspension to augment paroxetine bioavailability: In vitro characterization, ex vivo permeation, and pharmacokinetic evaluation in healthy human volunteers. Pharmaceutics. 13(11): 1869.
European Pharmacopoeia, Tablets, Strasbourg, France: European directorate for the quality of medicines (EDQM), 2016.
Fatima, A., Tirunagari, M., and Chilekampalli, D.T. 2021. Design, development and evaluation of instant release oral thin films of flunarizine. International Journal of Pharmaceutical Sciences Review and Research. 71(1): 137-142.
Göbel, A., Bassi da Silva, J., Cook, M., and Breitkreutz, J. 2021. Development of buccal film formulations and their mucoadhesive performance in biomimetic models. International Journal of Pharmaceutics. 610: 121233.
Iorgulescu, G. 2009. Saliva between normal and pathological. Important factors in determining systemic and oral health. Journal of Medicine and Life. 2(3): 303–307.
Irfan, M., Rabel, S., Bukhtar, Q., Qadir, M.I., Jabeen, F., and Khan, A. 2016. Orally disintegrating films: A modern expansion in drug delivery system. Saudi Pharmaceutical Journal. 24(5): 537-546.
Jillani, U., Mudassir, J., Arshad, M.S., Mehta, P., Alyassin, Y., Nazari, K., et al. 2022. Design and evaluation of agarose based buccal films containing zolmitriptan succinate: Application of physical and chemical enhancement approaches. Journal of Drug Delivery Science and Technology. 69: 103041.
Katewongsa, P., Lertsuphotvanit, N., and Phaechamud, T. 2017. Cetirizine dihydrochloride, beta-cyclodextrin inclusion complex by ethanol kneading for taste masking. Indian Journal of Pharmaceutical Sciences. 9(5): 758-767.
Kumar, R.S., Kumari, A., Latha, B.K., and Raj, P. 2019. Design, optimization and evaluation of cetrizine dihydrochloride fast dissolving films employing starch tartrate–a novel superdisintegrant. International Journal of Current Pharmaceutical Research. 11(6): 75-86.
Kumar, A., Sharma, P.K., and Ali, A. 2013. HPMC/CMC based fast dissolvable oral films of an anxiolytic: In vitro drug release and texture analysis. International Journal of Drug Delivery. 5: 344-352
Lai, K.L., Fang, Y., Han, H., Li, Q., Zhang, S., Li, H.Y., et al. 2018. Orally-dissolving film for sublingual and buccal delivery of ropinirole. Colloids and Surfaces B: Biointerfaces. 163: 9–18.
Ouda, G.I., Dahmash, E.Z., Alyami, H., and Iyire, A. 2020. A novel technique to improve drug loading capacity of fast/extended release orally dissolving films with potential for paediatric and geriatric drug delivery. AAPS PharmSciTech. 21(4):126.
Paczkowska, M., Mizera, M., Lewandowska, K., Kozak, M., Miklaszewski, A., and Cielecka-Piontek, J. 2018. Effects of inclusion of cetirizine hydrochloride in β-cyclodextrin. Journal of Inclusion Phenomena and Macrocyclic Chemistry. 91(3): 149–159.
Pamlényi, K., Kristó, K., Jójárt-Laczkovich, O., and Regdon, G.Jr. 2021. Formulation and optimization of sodium alginate polymer film as a buccal mucoadhesive drug delivery system containing cetirizine dihydrochloride. Pharmaceutics. 13(5): 619.
Patel, D.M., Patel, D.J., and Darji, P.J. 2016. Formulation and evaluation of fast-dissolving film of cetirizine and dextromethorphan. International Journal of Pharmaceutical Sciences and Nanotechnology. 9(3): 3305–3311.
Phalguna, Y., Pasupulati, H., and Rudra. S. 2019. Formulation, characterization and in-vitro evaluation of fast dissolving oral films of cetirizine HCl. Journal of Drug Delivery and Therapeutics. 9(4-A): 122-125.
Rakić, T., Kasagić-Vujanović, I., Jovanović, M, Jančić-Stojanović, B., and Ivanović D. 2014. Comparison of full factorial design, central composite design, and Box-Behnken design in chromatographic method development for the determination of fluconazole and its impurities. Analytical Letters. 47(8): 1334-1347.
Rani, K.C., Parfati, N., Aryani, N.L.D., Winantari, A.N., Fitriani, E.W., Pradana, A.T., et al. 2021. Development, evaluation, and molecular docking of oral dissolving film of atenolol. Pharmaceutics. 13: 1727.
Sevinç Özakar, R., and Özakar, E. 2021. Current overview of oral thin films. Turkish Journal of Pharmaceutical Sciences. 18(1): 111–121.
Sievens-Figueroa, L., Bhakay, A., Jerez-Rozo, J.I., Pandya, N., Romañach, R.J., Michniak-Kohn, B., et al. 2012. Preparation and characterization of hydroxypropyl methyl cellulose films containing stable BCS Class II drug nanoparticles for pharmaceutical applications. International Journal of Pharmaceutics. 423(2): 496–508.
Souri, E., Hatami, A., Shabani Ravari, N., Alvandifar, F., and Barazandeh Tehrani, M. 2013. Validating a stability-indicating HPLC method for kinetic study of cetirizine degradation in acidic and oxidative conditions. Iranian Journal of Pharmaceutical Research. 12(2): 287–294.
Sri rekha, M., Shaheda, S.K., Mahathi, K., Praveen, P., Prathama, B., and Seetha Devi, A. 2014. Novel oral drug delivery system: fast dissolving buccal films. American Journal of Pharmacy and Health Research. 5: 1-40.
Takeuchi, Y., Umemura, K., Tahara, K., and Takeuchi, H. 2018. Formulation design of hydroxypropyl cellulose films for use as orally disintegrating dosage forms. Journal of Drug Delivery Science and Technology. 46: 93-100
Takeuchi, Y., Ikeda, N., Tahara, K., and Takeuchi, H. 2020. Mechanical characteristics of orally disintegrating films: Comparison of folding endurance and tensile properties. International Journal of Pharmaceutics, 589, 119876.
The United State Pharmacopeial Convention. The United States pharmacopeia 2018: USP 41; The national formulary: NF 36. United Book Press, Maryland, 2018.
U.S. Food and Drug Administration (FDA), Guidance for industry: orally disintegrating tablets, 2008.
Wasilewska, K. and Winnicka, K. 2019. How to assess orodispersible film quality? A review of applied methods and their modifications. Acta Pharmaceutica. 69(2): 155–176.
Yir-Erong, B., Bayor, M.T., Ayensu, I., Gbedema, S.Y., and Boateng, J.S. 2019. Oral thin films as a remedy for noncompliance in pediatric and geriatric patients. Therapeutic Delivery. 10(7): 443–464.
OPEN access freely available online
Natural and Life Sciences Communications
Chiang Mai University, Thailand. https://cmuj.cmu.ac.th
Kamchai Saepang1, 2, Tasana Pitaksuteepong3, Benjaporn Buranrat2, 4, and Supavadee Boontha1, 2,*
1 School of Pharmaceutical Sciences, University of Phayao, Phayao 56000, Thailand.
2 Research Group in Herbal and Development of Formulation and Delivery Systems for Elderly Adults and Cancer Treatment, School of Pharmaceutical Sciences, University of Phayao, Phayao 56000, Thailand.
3 Department of Pharmaceutical Technology, Faculty of Pharmaceutical Sciences, Naresuan University, Phitsanulok 65000, Thailand.
4 Faculty of Medicine, Mahasarakham University, Maha Sarakham 44000, Thailand.
Corresponding author: Supavadee Boontha, E-mail: supavadee.bo@up.ac.th
Total Article Views
Editor: Wipawadee Yooin,
Chiang Mai University, Thailand
Article history:
Received: June 4, 2023;
Revised: November 12, 2023;
Accepted: December 14, 2023;
Online First: December 21, 2023

