
Ultrasonication on Collagen Yield, Physiochemical and Structural Properties from Seabass (Lates Calcarifer) Scales as Affected by Pretreatment and Extraction Conditions
Sri Charan Bindu Bavisetty, Supatra Karnjanapratum *, Jaydeep Dave, Daniel Tua Purba, Tanaji Kudre, Wahyu Haryati Maser, Nur Maiyah, Passakorn Kingwascharapong, and Ali Muhammed Moula Ali*Published Date : December 13, 2023
DOI : https://doi.org/10.12982/NLSC.2024.003
Journal Issues : Number 1, January-March 2024
Abstract This research explored the use of ultrasound as a pretreatment and extraction assisted process for extraction of collagen from seabass scales. Both acid-soluble (AC) and pepsin-soluble collagens (PC) were extracted with ultrasonication at different extraction times (24 and 48 h). Ultrasound pretreatment (PU) and ultrasound-assisted extraction (UE) notably improved AC extraction yields over 48 hours, registering 2.12% and 4.62%, respectively. The highest yield for PC, 21.85%, was achieved with 48 hours of ultrasound assisted extraction. Protein analysis verified the extracted collagens as type I, indicated by the presence of specific α and β chains. Further, FTIR spectra confirmed collagen's presence in all samples through distinct amide band peaks. Interestingly, ultrasound processes marginally reduced AC's thermal stability while boosting PC's thermal stability, especially when combined with pepsin treatment. Moreover, CD spectroscopy showed the preserved native structure of collagen across all samples. The ultrasound method increased collagen extraction yields and retained its structural integrity. These outcomes are pivotal for advancing collagen extraction methods in the biomedical and food sectors.
Keywords: Seabass scales, Collagen, Ultrasonication, Molecular characterization, Physicochemical properties
Citation: Bavisetty, S.C.B., Karnjanapratum, S., Dave, J., Purba, D.T., Kudre, T., Maser, W.H., Nur Maiyah, N., Kingwascharapong, P., and Ali, A.M.M. 2024. Ultrasonication on collagen yield, physiochemical and structural properties from seabass (Lates Calcarifer) scales as affected by pretreatment and extraction conditions. Natural and Life Sciences Communications. 23(1): e2024003.
INTRODUCTION
Collagen, the most abundant protein in the animal world, is the keystone for numerous applications across biomedicine, cosmetics, food, and pharmaceutical industries. This fibrous protein, known for its exceptional mechanical properties and biocompatibility, is typically extracted from mammalian sources. Commercially, collagen is derived from porcine and bovine sources (Liu et al., 2007). However, zoonotic diseases, cultural restrictions, and sustainability concerns have heightened the interest in alternate resources like fish (Prommajak and Raviyan, 2010; Nguyen et al., 2020). Various studies have demonstrated fish skin as a sustainable source for collagen extraction (Ali et al., 2018; Petcharat et al., 2020).
Seabass (Lates calcarifer) is a popular fish species with a high demand in the food industry. Fish scales are a by-product of the fish processing industry, produced during descaling process (Ali et al., 2017). Generally, fish scales have been considered as low value products and discarded as waste, hence contributing to pollution. These scales are made of extracellular matrix, which demonstrated good water absorption and retention by 13.3% and 15%, respectively, and composed collagen and hydroxyapatite, which combine to form highly ordered collagen fibers with tightly cross-linked regions (Huang et al., 2016). In past decades, few studies have documented the extraction of collagen from fish scales using traditional methods i.e., acid soluble collagen (ASC) extraction and pepsin soluble collagen (PSC) extraction. Collagen from golden carp scales yielded 0.42 and 1.16 g 100 g-1 (dry weight basis), ASC and PSC, respectively (Ali et al., 2017). Furthermore, collagen extracted from silver carp fish scales produced successful yields (5.09 and 12.06%) when ASC and PSC were used (Wu et al., 2019). However, these ASC and PSC methods are time consuming and generate lower yield, hence, a significant amount of insoluble collagen is still retained (Ali et al., 2018). Furthermore, the extra cellular matrix of fish scales may hinder the penetration of solvents for efficient collagen extraction (Kameshwar et al., 2024). Thus, the industry and researchers pursue innovative techniques that ensure yield and quality.
The application of ultrasound can produce a cavitation effect that effectively disrupts the surface of the raw material, leading to an improvement in mass transfer rates and the penetration of solvents into and out of the material (Benjakul et al., 2019). This results in a higher yield of target compounds. Furthermore, the use of ultrasound also causes an increase in the swelling and softening of the raw material when combined with a solvent, which is beneficial for the extraction process (Ali et al., 2019). In addition to cavitation, ultrasound generates various mechanisms, such as agitation, turbulence, and particle collisions, that further enhance the extraction process.
Ultrasound technology has been recently explored in two capacities: as a pretreatment step to precondition the scales and as an active extraction method, known as Ultrasound-Assisted Extraction (UAE) (Shen et al., 2023). When used as a pretreatment, ultrasound relies on the cavitation effect, where rapid compression and rarefaction cycles create a microbubble (Petcharat et al., 2021). The subsequent collapse of these bubbles mechanically disrupts the fish tissues, making them more amenable for extraction. This preconditioning can enhance the efficiency of subsequent extraction processes. On the other hand, when ultrasound is incorporated directly into the extraction process or UAE, it not only preconditions the scales but also actively promotes the diffusion of solvents into the disrupted matrix (Pingret et al., 2013). This dual action disruption and diffusion enhancement has the potential to significantly increase the yield of extracted compounds (Sengkhamparn et al., 2019). Ali et al. (2019) have observed that ultrasound assisted direct in situ saponification process increase the yield of unsaponifiable matter (rich in squalene) from different fish livers. Furthermore, UAE reduced the extraction time and enhance the structural integrity of extracted compounds (Ali, Benjakul, et al., 2018).
This research aims to comprehensively explore both facets of ultrasound application in collagen extraction from seabass scales. By investigating the synergistic effects of ultrasound pretreatment and UAE, we endeavor to establish an optimized, eco-friendly, and high-yield protocol for collagen extraction. Comparisons with traditional methods will further elucidate the effectiveness of this innovative approach, paving the way for its potential industrial adoption and redefining standards in collagen extraction.
MATERIALS AND METHODS
Chemicals
All chemicals used were of analytical quality. Acetic acid and sodium hydroxide were sourced from Merck (Darmstadt, Germany). Porcine pepsin (EC3.4.23.1) and markers for protein molecular weight were supplied by Sigma Chemicals (St. Louis, MO, USA). Chemicals required for electrophoresis, such as sodium dodecylsulphate (SDS), N,N,N′,N′-tetramethylethylenediamine (TEMED), and Coomassie Blue R-250, were obtained from Bio-Rad Laboratories (Hercules, CA, USA).
Preparation of raw material
Fish scales of seabass were obtained from the fish processing industry around Bangkok. They were packed in ice with a ratio of 3:1 (ice to sample) and transported to the School of Food-Industry at King Mongkut’s Institute of Technology Ladkrabang. All scales were meticulously rinsed in a salt solution (0.5 % w/v) and stored at -20 ºC for future use. The scales were analyzed for their proximate composition following the method described by AOAC (AOAC, 2017).
Collagen extraction
The scales had been thawed under running tap water until their core temperature had reached 8-10 ºC. All the pretreatment, cleansing, and extraction steps were performed at 4 °C to ensure collagen didn't denature.
Traditional collagen extraction from scales
Scale Pretreatment
Scale pretreatment was carried out using the method described by Chuaychan et al. (2015) with minor changes. Before extraction, scales were treated with 0.1 M NaOH, keeping a scale/alkaline solution ratio of 1:10 (w/v) to remove non-collagen proteins. This mixture was stirred continuously for 8 hours, with the alkaline solution replaced every 4 hours. The scales were washed with iced water until they reached a neutral pH. Subsequently, these scales were demineralized using a 0.5 M EDTA salt solution with a pH of 7.4, in a 1:10 (w/v) ratio, and stirred for about 48 hours. The EDTA solution was replaced after the first 24 hours. After demineralization, scales were washed until the water was neutral in pH. The scales were then set on a screen for drainage and moved on to collagen extraction.
Acid soluble collagen (AC) preparation
The pretreated scales (100 g) were submerged in 0.5 M acetic acid, keeping a scale/acid solution ratio of 1:15 (w/v), and stirred for 48 hours. This mixture has then been strained using a two-layered cheesecloth. Collagen in the resultant liquid had been made to precipitate using 2.5 M NaCl with 0.05 M tris (hydroxymethyl) aminomethane (pH 7.0). The settled collagen was collected by centrifugation at 15,000 ×g for 1 hour at 4 °C in a refrigerated centrifuge (Model CR 22GIII, Hitachi, Tokyo, Japan). The resulting mass had been redissolved in a small amount of 0.5 M acetic acid and dialyzed first with 20 volumes of 0.1 M acetic acid for 24 hours and then with 20 volumes of distilled water for another 24 hours. The collagen thus obtained had been dried using a freeze-dryer (CoolSafe 55, ScanLaf A/S, Lynge, Denmark). The resulting AC extracted without ultrasonication was referred as “AC”.
Pepsin soluble collagen (PC) preparation
PC had been extracted from the leftover mass post-AC extraction. This residue had been soaked in 0.5 M acetic acid that contained 1% (w/w) of porcine pepsin (50 unit g-1 residue) maintaining a matter/solution ratio of 1:15 (w/v). The mixture was constantly stirred for 24 hours, followed by the filtration and precipitation processes previously mentioned. The settled substance had been dialyzed and then freeze-dried similarly. The resulting PC extracted without ultrasonication was referred as “PC”.
Impact of ultrasonication on extraction efficiency and characteristics of acid and pepsin soluble collagens from seabass scales
Ultrasonication was employed both in pre-treatment (PU) and extraction processes (UE). Traditional methods were followed to prepare the samples. The fish scales, once demineralized, were subjected to extraction either using acid or by including pepsin enzyme. For the acid-only approach, a 0.5 M acetic acid solution was utilized at a ratio of 1:15 (w/v). The pepsin enzyme method combined 1% of the enzyme with the acetic acid solution.
During the ultrasonic pre-treatment (PU), the mixture of the sample and acetic acid solution was exposed to 15 minutes of ultrasound using a Vibra-Cell reactor (Sonics & Material, Inc, Newton, CT, USA) equipped with a 25 mm diameter flat-tip probe. This reaction was conducted at settings of 750 W power, 20 kHz frequency, 80% amplitude, using a pulsing sequence of 10 seconds on and 5 seconds off, all while keeping the solution cool with an ice bath. The mixture was also stirred continuously at 4 °C for 24 and 48 hours using an overhead stirrer from Germany. The resulting collagens extracted with ultrasound pretreatment process (PU) at 24 and 48 h of extraction hour were referred as “UP24” and “UP48”, respectively.
During the extraction aided by ultrasonication (UE), the samples underwent 15 minutes of ultrasound treatment every 12 hours under similar settings. They were then extracted at 4 °C for 24 and 48 hours while continuously stirring. Post-extraction, conventional methods were applied to the samples. The resulting collagens extracted with ultrasound-assisted extraction (UE) at 24 and 48 h of extraction hour were referred as “UE24” and “UE48”, respectively.
Characterizations
Both collagens, AC and PC from scales, extracted by PU and UE were calculated for yield and protein patterns.
Yield
The yield of AC and PC were calculated based on the wet weight of the initial scales obtained after pretreatment according to Eq. (1):

Collagen protein patterns
The protein pattern of the collagens was analyzed using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as per Laemmli (1970) with modifications by Ali et al. (2017). After dissolving the collagens in 5% SDS and heating them at 85 °C for 60 minutes, 18 mg protein samples (measured via the Biuret method) were added to a gel made of 4% stacking and 7.5% running components. Electrophoresis was run at 15 mA/plate current, after which the gel underwent staining processes. A high molecular weight protein marker was used to identify the molecular weight of collagen. ImageJ software (ImageJ 1.42q, National Institutes of Health, Bethesda, MD, USA) was used to determine the protein band intensity on the gel.
Analyses
The ultrasonication conditions, from both PU and UE, provided acid (ACPU24, ACUE48) and pepsin soluble collagens (PCPU48, PCUE48) with high yield and less adverse effect on collagen protein pattern were then selected and used for preparing the collagens for further analyses in comparison with the controls (AC and PC).
Amino acid compositions
Collagens underwent acid hydrolysis using a 4 M methanesulfonic acid solution enriched with 0.2% (v/v) 3-2(2-aminoethyl) indole, conducted under vacuum at 115 °C for a day. Post hydrolysis, activated charcoal was utilized to clarify the samples, which were then neutralized with NaOH. The resultant mixture was then adjusted to the desired volume using a 0.2 M citrate buffer with pH 2.2. Small portions (0.02-0.06 mL) of the samples were evaluated with an amino acid analyzer (model MLC-703, Atto Co., Tokyo, Japan), post a ninhydrin-based post-column derivatization step. All amino acid assessments were conducted twice.
FTIR spectroscopy
FTIR spectra of AC and PC were determined according to Ali et al., (2018) using an FTIR spectrometer, Model Equinox 55, spectra were taken from freeze-dried samples placed in a specialized crystal cell, spanning a wavenumber range of 650–4000 cm-1. The background was the spectrum of an empty cell. OPUS 3.0 software processed the spectral data.
Circular dichroism (CD) spectroscopy
Circular dichroism of extracted collagen samples was measured according to Akita et al. (2020). Samples were dissolved in 0.5 M acetic acid, stirred continuously for 4 h and centrifuged at 10,000 ×g for 5 min at 4 °C. A final concentration of 2.0× 10-4 g ml-1 was used for analysis using spectrometer (JASCO J-801, Jasco Corp, Tokyo, Japan) at wavelengths between 190–240 nm. The spectral data of 0.5 M acetic acid served as a blank. Results were presented as molar residual ellipticity.
DSC analysis
Samples were prepared for DSC according to Wu et al. (2019). After dissolving in deionized water, samples were weighed and sealed in aluminum pans. They were scanned from 25–45 °C at a 1 °C/min rate on a DSC7 model (Perkin–Elmer, Norwalk, CA, USA). The DSC thermogram's peak areas and endothermic peaks provided data on denaturation enthalpy (ΔH) and maximum transition temperature (Tmax).
Zeta potential
AC and PC were mixed in 0.05 M acetic acid (w/v) to reach a 0.04% final concentration. These solutions were constantly mixed at 4 °C using a magnetic stirrer until fully dissolved. The dissolved solution, totaling twenty milliliters, was then introduced to the auto titrator (BI-ZTU model from Brookhaven Instruments Co., Holtsville, NY, USA). Here, pH levels between 2 and 12 were set using either 1.0 M nitric acid or 1.0 M KOH. The zeta potential of the sample was measured using the ZetaPALs analyzer (Brookhaven Instruments Co., Holtsville, NY, USA).
Collagen solubility
Solution preparation
Collagen solubilities were tested according to Tan and Chang (2018) with minor adjustments. Collagens were dissolved in 0.5 M acetic acid, achieving a 3 mg ml-1 concentration, and mixed until fully dissolved at 4 °C.
Influence of pH on solubility
A solution (8 ml) was adjusted for pH using either 6 N NaOH or 6 N HCl, creating a pH 1-10 range. Following centrifugation, protein content in the resultant supernatant was assessed using the Lowry (1951) method suggested by Ali, Benjakul, et al. (2018). Comparative solubility was computed against the highest solubility pH.
Statistical assessment
All experiments were repeated thrice with three sample sets. ANOVA was applied to the data. The Duncan’s multiple range test was used to compare the means using SPSS 11.0 (SPSS 11.0 for Windows, SPSS Inc., Chicago, IL, USA).
RESULTS
Extraction yield
The extraction yield of collagen from various treatments was presented in Table 1. In the case of acid-soluble collagen treatments, the conventional method (AC) yielded 0.87% ± 0.23% collagen. When ultrasound pretreatment was applied for 24 hours (ACPU24), there was a slight increase in yield to 1.04% ± 0.59%. A more substantial increase was observed with a 48-hour ultrasound pretreatment (ACPU48), resulting in a yield of 2.12% ± 0.40%. Ultrasound-assisted extraction combined with 24-hour acid treatment (ACUE24) yielded 2.50% ± 0.83%, while the highest yield was achieved with ACUE48, where ultrasound-assisted extraction for 48 hours yielded 4.62% ± 0.23% collagen.
For pepsin-soluble collagen treatments, the control (PC) yielded 5.02% ± 0.40% collagen, and ultrasound pretreatment for 24 hours (PCPU24) increased the yield slightly to 5.24% ± 0.49%. However, a significant improvement was seen with a 48-hour ultrasound pretreatment (PCPU48), resulting in a yield of 10.39% ± 0.25%. Ultrasound-assisted extraction combined with 24-hour pepsin treatment (PCUE24) yielded 10.94% ± 0.23%, and the highest yield was obtained with PCUE48, where ultrasound-assisted extraction for 48 hours yielded 21.85% ± 0.62% collagen.
Table 1. Extraction yield of acid and pepsin soluble collagens from seabass scales extracted with ultrasound pretreatment and ultrasound-assisted extraction.
|
Treatment |
Yield (%) |
|
AC |
0.87 ± 0.23a |
|
ACPU24 |
1.04 ± 0.59a |
|
ACPU48 |
2.12 ± 0.40b |
|
ACUE24 |
2.50 ± 0.83c |
|
ACUE48 |
4.62 ± 0.23d |
|
PC |
5.02 ± 0.40e |
|
PCPU24 |
5.24 ± 0.49f |
|
PCPU48 |
10.39 ± 0.25h |
|
PCUE24 |
10.94 ± 0.23i |
|
PCUE48 |
21.85 ± 0.62j |
Note: Values are given as mean ± SD (n = 3), AC- Acid soluble collagen (Control), ACPU24- Acid soluble collagen with ultrasound pretreatment for 24 hours, ACPU48- Acid soluble collagen with ultrasound pretreatment for 48 hours, ACUE24- Acid soluble collagen with ultrasound assisted extraction for 24 hours, ACUE48- Acid soluble collagen with ultrasound assisted extraction for 48 hours, PC- Pepsin soluble collagen using (Control), PCPU24- Pepsin soluble collagen using with ultrasound pretreatment for 24 hours, PCPU48- Pepsin soluble collagen with ultrasound pretreatment for 48 hours, PCUE24- Pepsin soluble collagen with ultrasound assisted extraction for 24 hours, PCUE48- Pepsin soluble collagen with ultrasound assisted extraction for 48 hours. Superscript letters show the significant difference (P < 0.05) between the treatments.
Protein patterns
From the electrophoretic profiles, all collagens exhibited the presence of α1-, α2-, and β-chains (dimer) as principal constituents (Figure 1). The intensity patterns of α1 and α2 bands in AC, ACPU24, and ACUE24 appeared consistent. However, for ACPU48 and ACUE48, there was a discernible decline in band intensities relative to AC. Relative to PC, the α1 and α2 chain intensities in PCPU24, PCPU48, PCUE24, and PCUE48 appeared marginally diminished, yet the α-chain intensities were notably more pronounced than those observed in AC and its ultrasonically treated counterparts.
All collagen samples were observed with high molecular weight γ-chains (trimer) and β-chains (dimer).The intensity of β-chain was higher in ACPU48 and PCUE48 compared to the controls (AC and PC) (Figure 1). The higher intensity of β-chain bands in ACPU48 and PCUE48 may suggest that more collagen was successfully extracted from the seabass scales. These results are in line with the yield of collagen presented in Table 1. Negligible low molecular weight proteins were observed.
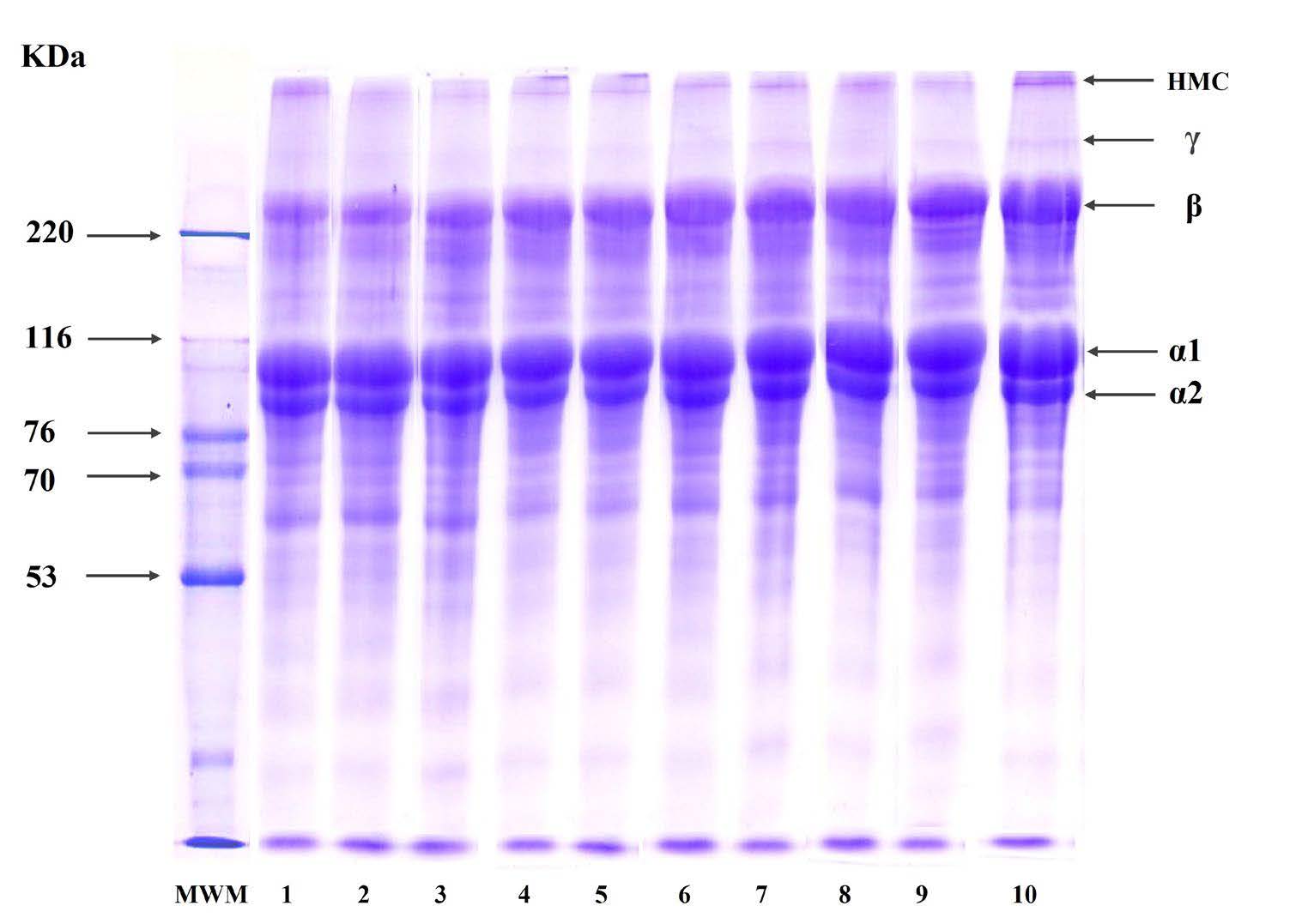
Figure 1. SDS-PAGE patterns of acid and pepsin soluble collagens from seabass scales extracted with ultrasound pretreatment and ultrasound-assisted extraction. 1. AC- Acid soluble collagen (Control), 2. ACPU24- Acid soluble collagen with ultrasound pretreatment for 24 hours, 3. ACPU48- Acid soluble collagen with ultrasound pretreatment for 48 hours, 4. ACUE24- Acid soluble collagen with ultrasound assisted extraction for 24 hours, 5. ACUE48- Acid soluble collagen with ultrasound assisted extraction for 48 hours, 6. PC- Pepsin soluble collagen using (Control), 7. PCPU24- Pepsin soluble collagen using with ultrasound pretreatment for 24 hours, 8. PCPU48- Pepsin soluble collagen with ultrasound pretreatment for 48 hours, 9. PCUE24- Pepsin soluble collagen with ultrasound assisted extraction for 24 hours, 10. PCUE48- Pepsin soluble collagen with ultrasound assisted extraction for 48 hours.
Amino acid composition
Table 2 shows the amino acid compositions of acid-soluble collagen (AC) and pepsin-soluble collagen (PC) derived from seabass scales using conventional extraction, ultrasound-assisted pretreatment (ACPU24 and PCPU48), and ultrasound-assisted extraction (ACUE48 and PCUE48). Each value was expressed as residues per 1,000 residues, providing a standardized metric for comparing the compositions across extraction techniques.
All the collagen samples had glycine as the most abundant amino acid, irrespective of the extraction method, ranging from 324 to 336 residues/1,000 residues. In this study, pepsin-soluble collagen variants, specifically the PC and PCUE48, exhibited marginally higher glycine residues (332-336 residues/1,000 residues) than their acid-soluble counterparts, AC and ACUE48 (325-327 residues/1,000 residues). Furthermore, imino acids, namely proline and hydroxyproline, also exhibited significant differences based on the extraction technique. Notably, the PCUE48 reached up to 203 residues/1,000 residues, a slight increase when compared to the AC (194 residues/1,000 residues). Conversely, amino acids like isoleucine experienced a decline, especially evident in the PCPU48 and PCUE48.
Table 2. Amino acid composition of acid and pepsin soluble collagens from seabass scales extracted with selected ultrasonication conditions.
|
Amino acid |
AC |
ACPU24 |
ACUE48 |
PC |
PCPU48 |
PCUE48 |
|
Alanine |
111 |
118 |
119 |
121 |
122 |
118 |
|
Arginine |
53 |
53 |
53 |
53 |
53 |
53 |
|
Aspartic acid/asparagine |
51 |
50 |
50 |
49 |
50 |
50 |
|
Cysteine |
0 |
0 |
0 |
0 |
0 |
0 |
|
Glutamine/glutamic acid |
72 |
70 |
70 |
68 |
68 |
68 |
|
Glycine |
327 |
325 |
324 |
336 |
332 |
332 |
|
Histidine |
4 |
4 |
4 |
4 |
4 |
4 |
|
Isoleucine |
13 |
13 |
13 |
11 |
10 |
09 |
|
Leucine |
25 |
25 |
24 |
22 |
23 |
23 |
|
Lysine |
28 |
28 |
27 |
26 |
25 |
25 |
|
Hydroxylysine |
7 |
7 |
7 |
6 |
5 |
5 |
|
Methionine |
12 |
12 |
12 |
12 |
12 |
12 |
|
Phenylalanine |
14 |
14 |
14 |
13 |
13 |
13 |
|
Hydroxyproline |
78 |
77 |
77 |
79 |
80 |
81 |
|
Proline |
116 |
117 |
118 |
118 |
119 |
121 |
|
Serine |
37 |
37 |
37 |
35 |
36 |
37 |
|
Threonine |
25 |
24 |
25 |
23 |
23 |
23 |
|
Tyrosine |
4 |
4 |
4 |
3 |
3 |
3 |
|
Valine |
23 |
22 |
22 |
21 |
22 |
23 |
|
Total residues |
1,000 |
1,000 |
1,000 |
1,000 |
1,000 |
1,000 |
|
Imino acid |
194 |
194 |
195 |
197 |
202 |
203 |
Note: Value was expressed as residues per 1,000 residues. AC- Acid soluble collagen (Control), ACPU24- Acid soluble collagen with ultrasound pretreatment for 24 hours, ACUE48- Acid soluble collagen with ultrasound assisted extraction for 48 hours, PC- Pepsin soluble collagen using (Control), PCPU48- Pepsin soluble collagen with ultrasound pretreatment for 48 hours, PCUE48- Pepsin soluble collagen with ultrasound assisted extraction for 48 hours.
FTIR Spectra
FTIR spectra for AC, PC, ACPU24, ACUE48, PCPU48, and PCUE48 revealed distinct peaks corresponding to amide groups A, B, I, II, and III (Figure 2). The vibrational bands for amide I, II, and III, corresponding to collagen, are associated with 1,600-1,700 cm-1, 1,500-1,600 cm-1, and 1,200-1,300 cm-1, respectively (Ali et al., 2017). Specifically, the amide I band, indicative of C=O stretching, was detected at specific wavenumbers for each collagen type. The slightly lower wavenumbers in PC, PCPU48, and PCUE48. For the amide II band, which corresponds to N-H bending, variances were noted in the wavenumbers between PC derivatives and AC types. Amide III, a combination of C-N stretching and N-H deformation, remained consistent across all collagen types at a wavenumber of 1,236 cm-1. Furthermore, the wavenumber shifts in the amide A and amide B bands was observed as shown in Figure 2.
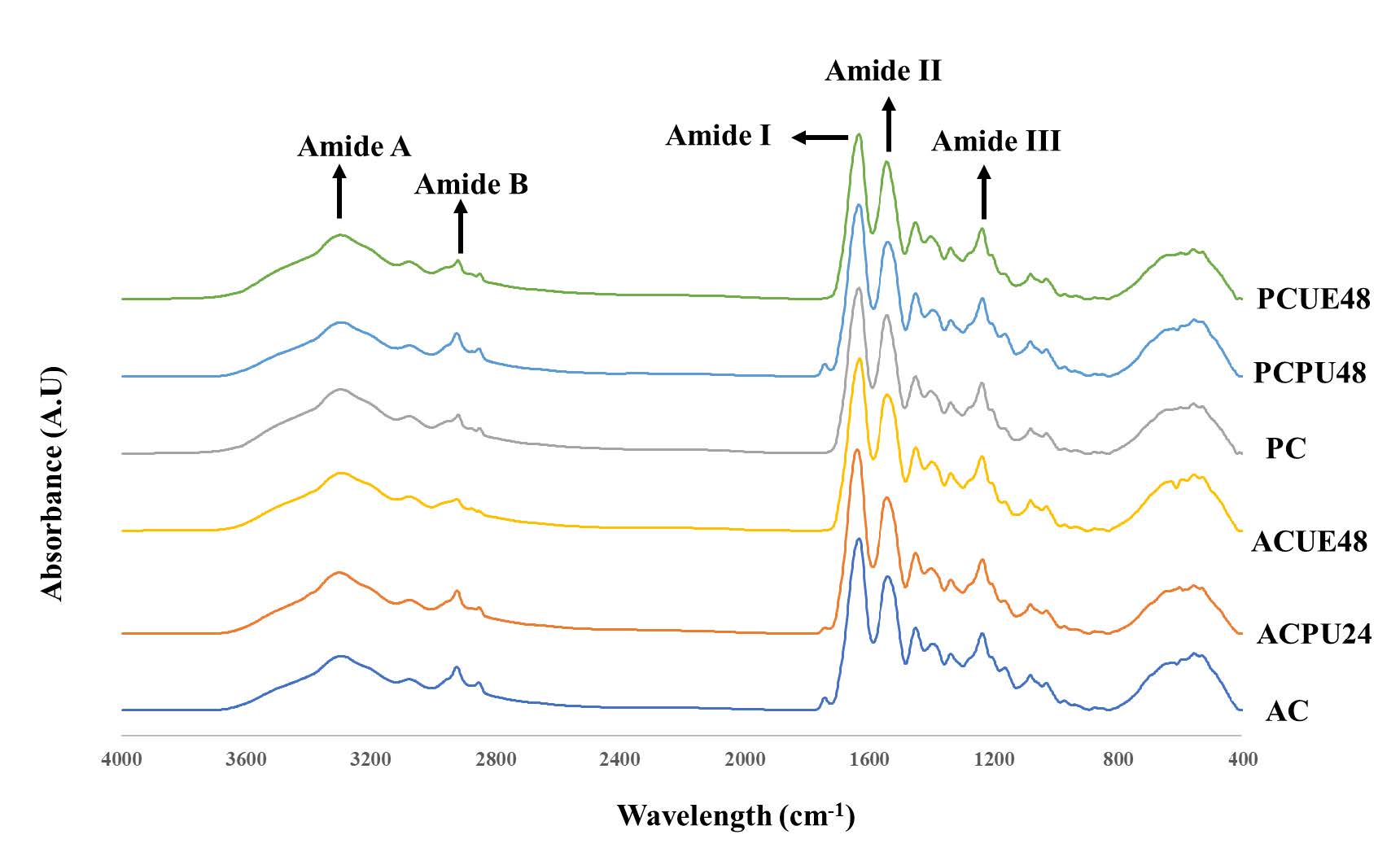
Figure 2. Fourier transform infrared spectra of acid and pepsin soluble collagens from seabass scales extracted with selected ultrasonication conditions. AC- Acid soluble collagen (Control), ACPU24- Acid soluble collagen with ultrasound pretreatment for 24 hours, ACUE48- Acid soluble collagen with ultrasound assisted extraction for 48 hours, PC-pepsin soluble collagen (Control), PCPU48- Pepsin soluble collagen with ultrasound pretreatment for 48 hours, PCUE48- Pepsin soluble collagen with ultrasound assisted extraction for 48 hours.
Thermal transition
The thermal properties of collagen samples from seabass scales were assessed through their Tmax (peak temperature) and ΔH (enthalpy change) determinations. Table 3 reveals variations in these thermal attributes. AC recorded a Tmax at 37.24 °C, signifying its thermal resilience within the examined temperature spectrum. The ultrasound-pretreated sample, ACPU24, and the ultrasound-assisted extract, ACUE48, registered marginally reduced Tmax values at 36.79 °C and 36.13 °C, respectively. On the other hand, samples subjected to pepsin, namely PC, PCPU48, and PCUE48, documented elevated Tmax readings at 39.61 °C, 40.12 °C, and 41.37 °C, respectively. Ultrasound integration during pre-treatment (PCPU48) seemingly augmented this resilience, given its Tmax of 40.12 °C. Remarkably, PCUE48, with its top Tmax reading of 41.37 °C.
Table 3. Maximum transition temperature (Tmax) and total denaturation enthalpy (ΔH) of acid and pepsin soluble collagens from seabass scales extracted with selected ultrasonication conditions.
|
Sample |
Tmax (°C) |
ΔH (J/g) |
|
AC |
37.24 ± 0.84a |
0.71 ± 0.09a |
|
ACPU24 |
36.79 ± 0.39a |
0.68 ± 0.11a |
|
ACUE48 |
36.13 ± 0.65a |
0.63 ± 0.02a |
|
PC |
39.61± 0.81b |
0.87 ± 0.05b |
|
PCPU48 |
40.12 ± 0.43b |
0.93 ± 0.17b |
|
PCUE48 |
41.37 ± 0.43b |
1.18 ± 0.23c |
Note: Values are given as mean ± SD (n = 3). AC- Acid soluble collagen (Control), ACPU24- Acid soluble collagen with ultrasound pretreatment for 24 hours, ACUE48- Acid soluble collagen with ultrasound assisted extraction for 48 hours, PC- Pepsin soluble collagen using (Control), PCPU48- Pepsin soluble collagen with ultrasound pretreatment for 48 hours, PCUE48- Pepsin soluble collagen with ultrasound assisted extraction for 48 hours. Superscript letters (a-c) show the significant difference (P < 0.05) between selected collagen samples.
CD spectroscopy
Minor variations in the rotatory maxima were observed among the different samples. For instance, PCPU48 had a slightly lower rotatory maximum of 219.8 nm compared to PCUE48 (221 nm), while PC and AC showed rotatory maxima at 219.6 nm and 217.8 nm, respectively. Additionally, the CD spectra of pepsin with ultrasound were higher than those without ultrasound.
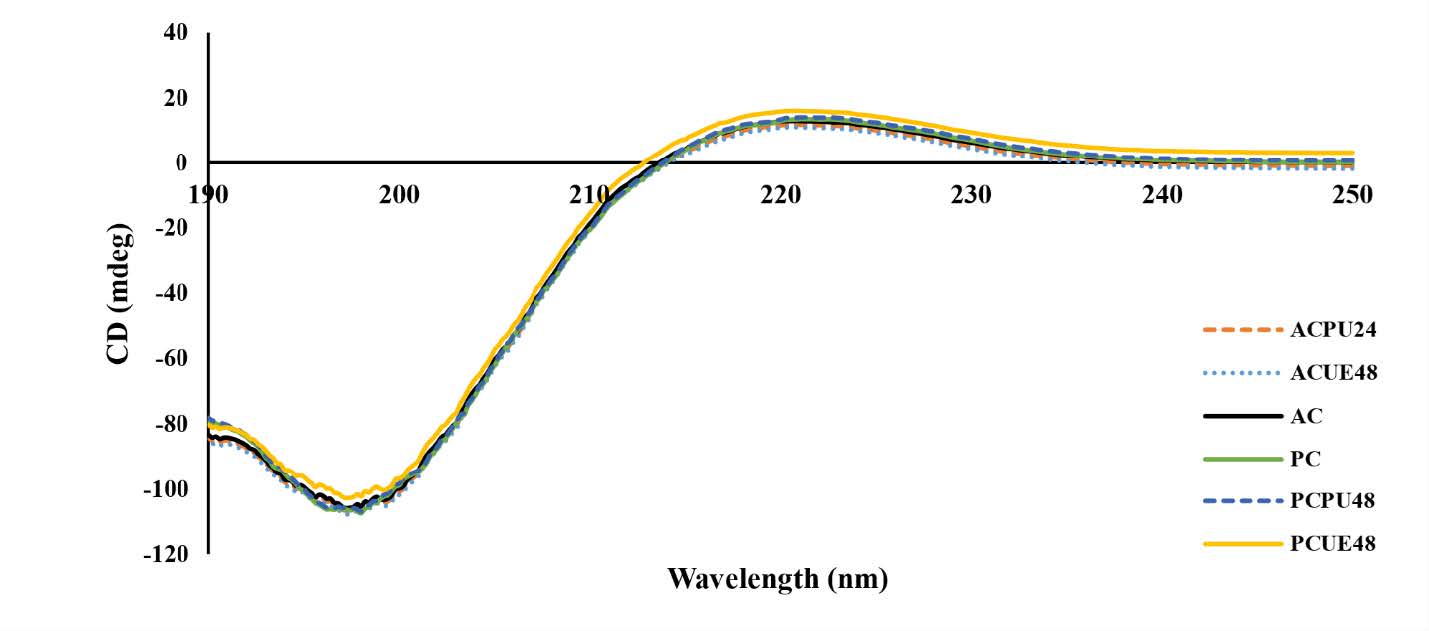
Figure 3. Circular dichroism spectra of acid and pepsin soluble collagens from seabass scales extracted with selected ultrasonication conditions. AC- Acid soluble collagen (Control), ACPU24- Acid soluble collagen with ultrasound pretreatment for 24 hours, ACUE48- Acid soluble collagen with ultrasound assisted extraction for 48 hours, PC-pepsin soluble collagen (Control), PCPU48- Pepsin soluble collagen with ultrasound pretreatment for 48 hours, PCUE48- Pepsin soluble collagen with ultrasound assisted extraction for 48 hours.
Zeta potential
The Zeta potential of the seabass scale under different pH conditions is depicted in Figure 4. The zeta potential of collagen extracted from seabass scales exhibited a net positive charge in acidic pHs (2-6). As the pH increased, the charge of collagen decreased, eventually reaching the isoelectric point within the pH range of 4.61-5.81. determined to be 4.61, 4.73, 5.23, 5.48, 5.62, and 5.81, respectively, based on the observation of zero surface net charge, indicating their respective isoelectric points (Ahmed et al., 2019).

Figure 4. Zeta potential of acid and pepsin soluble collagens from seabass scales extracted with selected ultrasonication conditions at different pHs. AC- Acid soluble collagen (Control), ACPU24- Acid soluble collagen with ultrasound pretreatment for 24 hours, ACUE48- Acid soluble collagen with ultrasound assisted extraction for 48 hours, PC-pepsin soluble collagen (Control), PCPU48- Pepsin soluble collagen with ultrasound pretreatment for 48 hours, PCUE48- Pepsin soluble collagen with ultrasound assisted extraction for 48 hours. Bars represent the standard derivation (n = 3).
The pIs were similar to collagen derived from seabass scales (Chuaychan et al., 2015). The zeta potential of PC, PCPU48, and PCUE48 exhibited higher pIs compared to ACUE48, ACPU24, and AC.
Solubility
The solubility of ACUE48, ACPU24, AC, PC, PCPU48, and PCUE48 derived from seabass scales was determined at various pH levels, as presented in Figure 5. Different solubility patterns were observed among all the samples, indicating a consistent response to changes in pH. AC, PCPU48, and PCUE48 exhibited higher solubility than ACUE48, ACPU24, and AC.
The solubility of collagen derived from seabass scales was found to be highest in the pH range of 1-3, which is consistent with previous studies involving acid-soluble collagen and pepsin-soluble collagen from seabass scales (Chuaychan et al., 2015). Additionally, the data consistently shows that ASC exhibited lower solubility compared to PSC across various pH levels. A slight increase in solubility was observed at pH 8 and 9.The solubility of collagen derived from seabass scales was found to be highest in the pH range of 1-3, which is consistent with previous studies involving acid-soluble collagen and pepsin-soluble collagen from seabass scales (Chuaychan et al., 2015).

Figure 5. Relative solubility of acid and pepsin soluble collagens from seabass scales extracted with selected ultrasonication conditions at different pHs. AC- Acid soluble collagen (Control), ACPU24- Acid soluble collagen with ultrasound pretreatment for 24 hours, ACUE48- Acid soluble collagen with ultrasound assisted extraction for 48 hours, PC-pepsin soluble collagen (Control), PCPU48- Pepsin soluble collagen with ultrasound pretreatment for 48 hours, PCUE48- Pepsin soluble collagen with ultrasound assisted extraction for 48 hours. Bars represent the standard derivation (n = 3).
DISCUSSION
For acid-soluble collagen extraction, the ultrasound pretreatment applied at 24 hours (ACPU24), slightly improved the collagen yield as compared to control (AC). This slight improvement was because of the cavitation effect of ultrasound to loosen the pretreated scales. Similar results were observed by Zou et al. (2017) during collagen extraction from softshell turtles using ultrasound. However, a more substantial increase was marked with a more prolonged ultrasound pretreatment. Ali, Kishimura, et al. (2018) also observed that the longer ultrasonication time rendered a higher yield for golden carp skin collagen. The substantial increase in yield compared to the control indicates ultrasound's positive effect during extraction. Thus, ultrasound with cavitation effect more likely loosened the scales. Therefore, acetic acid could penetrate through the scales more effectively and was able to extract collagen more potentially (Pongsetkul et al., 2018b; Shaik et al., 2021). For pepsin-soluble collagen extraction, pepsin has been known to increase collagen extraction by cleavage of the telopeptide region (Ali, Kishimura, et al., 2018; Gao et al., 2018). The highest yield among all the treatments was achieved with PCUE48, indicates that the combination of ultrasound-assisted extraction and extended pepsin treatment (48 hours) is highly effective in maximizing collagen yield.
All extracted collagen samples exhibited approximate 2:1 ratio of α1 to α2 chains, hence were categorized as type I collagen, as depicted by Ali et al. (2017). The consistency of intensity patterns of α1 and α2 bands in AC, ACPU24, and ACUE24 have confirmed that the 24-hour ultrasonic pretreatments or ultrasound-assisted extractions did not markedly alter the protein's band intensities. However, the decline in band intensities for ACPU48 and ACUE48, might be due to prolonged ultrasonication. Ma et al. (2018) also observed the degradation of α1 and α2 chains in AC from seabass skin upon prolonged ultrasonication. The presence of high molecular weight γ-chains (trimer) and β-chains (dimer) indicates that extracted collagen contained high amounts of covalent inter-molecular cross (Sun et al., 2017). The higher intensity of β-chain in ACPU48 and PCUE48 confirmed that the ultrasound disrupts the matrix of fish scales, making it easier to extract collagen through higher acid and enzyme penetration (Gao, 2021).
In this study, all collagen samples had glycine as the most abundant amino acid. Glycine constitutes one-third of the total amino acid residues, excluding the first fourteen residues from N-terminal and the last ten residues from the C-terminal in a polypeptide chain (Ahmed et al., 2022). Glycine plays a vital role in collagen stabilization by forming inter-chain hydrogen bonds, ensuring the integrity of the α-chain (Pozzolini et al., 2020). The pyrrolidine ring present in the imino acids, namely proline and hydroxyproline is fundamental to the stabilization of the collagen triple helix, facilitating inter-chain hydrogen bonds (Wu et al., 2019). Ali, Kishimura, et al. (2018) also observed that the enrichment of imino acids in ultrasoundassisted samples could suggest a more efficient or selective extraction or potentially better preservation of these residues. The decline in isolucine might hint at potential degradation or selective extraction of this amino acid during the ultrasound-assisted extraction process.
All collagen samples have shown the similar FTIR spectra patterns observed in other aquatic skin collagens, such as golden carp (Ali, Benjakul, et al., 2018) and scales of silver carp (Wu et al., 2019). The slightly lower wavenumbers in PC, PCPU48, and PCUE48 hint at enhanced hydrogen bonding in these samples, facilitating the triple helix structure's stabilization (Ahmed et al., 2022). For the amide II band, the higher intensity in PC variants might be due to removal of the non-helical region by pepsin likely influenced the elevated structural orderliness of collagen. Furthermore, the wavenumber shifts in the amide A and amide B bands, linked to N-H stretching and asymmetrical CH2 stretching, underscore the intricate intermolecular interactions within collagen and its triple helical configuration (Ali, Kishimura, et al., 2018).
In this study, the thermal properties of extracted collagen samples demonstrated that ultrasound interventions, whether pre-treatment or during extraction, marginally reduce thermal resilience relative to the acetic acid standard (Ali, Kishimura, et al., 2018). Pepsin's role in cleaving non-helical telopeptides might explain PC's superior Tmax over AC, presenting the collagen with a more structured and dense configuration, elevating its thermal robustness (Atef et al., 2020). The Tmax deviation between AC and its ultrasound variations was minimal, hinting that ultrasound might not critically modulate collagen's innate thermal characteristics (Ali, Kishimura, et al., 2018).
In the study of CD Spectra, the positive peaks indicate the presence of the triple helical conformation of collagen. The presence of these peaks suggests that the collagens derived from seabass scales maintained their native secondary structure (Pongsetkul et al., 2018a, Petcharat et al., 2021). The differences in the secondary conformation of collagens may be attributed to several factors, including the presence or absence of telopeptides and the effects of ultrasound treatment (Hong et al., 2019). Therefore, the slightly higher rotatory maximum observed for pepsin compared to acetic acid could be attributed to removing non-helical telopeptide regions during the extraction process (Ali et al., 2017). Additionally, the CD spectra of pepsin with ultrasound were higher than those without ultrasound. These values were comparable to those of collagen, indicating that ultrasound during collagen extraction influences pepsin to cleave the telopeptide region more effectively (Ali, Benjakul, et al., 2018).
The isoelectric points of ACUE48, ACPU24, AC, PC, PCPU48, and PCUE48 were determined to be 4.61, 4.73, 5.23, 5.48, 5.62, and 5.81, respectively, based on the observation of zero surface net charge, indicating their respective isoelectric points (Ahmed et al., 2019). The pIs were similar to collagen derived from seabass scales (Chuaychan et al., 2015). The zeta potential of PC, PCPU48, and PCUE48 exhibited higher pIs compared to ACUE48, ACPU24, and AC. This correlation might be attributed to the pI in the acidic pH range. The difference in the amino acid composition of the alpha chains is likely the result of pepsin cleaving the telopeptide region of the collagen molecule (Li et al., 2018). The charge of collagens is determined by the amino acids, which undergo protonation and deprotonation at the tested pH levels (Ali, Benjakul, et al., 2018). Regarding the impact of ultrasound treatment, the mentioned source suggests that ultrasonication does not significantly affect the pI of collagens. Collagens exhibit a negative charge at pH levels above the pI, and higher negative charge values are achieved with more basic pH levels. The variations in zeta potential profiles within the collagen molecule could be attributed to the distribution of amino acids, particularly on the surface of collagens (Indriani et al., 2022). The different distributions of amino acids, such as glutamic acid, lysine, aspartic acid, and specific sequences, may contribute to the differences in pI values among the collagens (Atef et al., 2020).
In the solubility study of collagen samples, AC, PCPU48, and PCUE48 exhibited higher solubility than ACUE48, ACPU24, and AC. Removing telopeptide regions could potentially impact the protonation of charged amino and carboxyl groups, thereby influencing the repulsion of molecules associated with different solubility (Chen et al., 2021). Solubility increases when the sample's pH differs from its isoelectric point (pI), whether it's lower or higher. This effect is due to the repulsion between protein chains caused by their positive or negative charges (Atef et al., 2020). Additionally, the data consistently shows that ASC exhibited lower solubility compared to PSC across various pH levels. This observation suggests that PSC likely has fewer cross-links than ASC. The decrease in solubility at higher pH levels could be attributed to hydrophobic interactions among collagen molecules (Ali, Benjakul, et al., 2018). A slight increase in solubility was observed at pH 8 and 9, which could be attributed to the repulsive effect of collagen molecules at pH values above their isoelectric point (Ahmed et al., 2019). The differences in the solubility of collagens as a function of pH can be attributed to variations in the molecular properties and conformations of the collagens (Ali et al., 2017).
CONCLUSION
The ultrasound-assisted extraction methodology has proven to be an effective technique for extracting collagen from seabass scales. The research highlighted that both ultrasound pretreatment and ultrasound-assisted extraction for 48 hours substantially boosted the extraction yield for acid-soluble collagen and pepsin-soluble collagen. Notably, the extracted collagens were identified as type I, evident from the presence of α1-, α2-, and β-chains. Furthermore, FTIR spectra confirmed the authenticity of collagen, while thermal transition analysis provided insights into the thermal stability of the collagens. Crucially, CD spectroscopy underscored the preservation of collagen's native triple helical structure across all samples. This study underscores the potential of ultrasound techniques in augmenting collagen extraction yields while preserving its inherent structure. The outcomes of this research offer significant contributions to both the biomedical and food sectors, pointing towards more efficient methods for collagen extraction and characterization.
ACKNOWLEDGMENTS
The authors would like to thank King Mongkut’s Institute of Technology Ladkrabang. Moula Ali and S.C.B. Bavisetty would like to acknowledge the support provided by the School of Food Industry, King Mongkut’s Institute of Technology Ladkrabang, Bangkok, Thailand (Grant #2564-02-07-003 and #2565-02-07-007), KRIS (King Mongkut’s Institute of Technology Ladkrabang, Research and Innovation Services) (Grant #KREF186318) and “National Science, Research and Innovation Fund”, Thailand (NSRF, #RE-KRIS/FF66/25 and RE-KRIS/FF66/27).
AUTHOR CONTRIBUTIONS
Sri Charan Bindu Bavisetty conducted research activities, investigating and writing the manuscript. Supatra Karnjanapratum work plan and critically improvising the manuscript. Jaydeep Dave conducted some experiments and wrote some sections of the manuscript. Daniel Tua Purba wrote some sections of the manuscript. Tanaji Kudre revised the manuscript with critical inputs. Wahyu Haryati Maser conducted some experiments. Nur Maiyah conducted some experiments. Passakorn Kingwascharapong revised the manuscript with critical inputs. Ali Muhammed Moula Ali work plan and designed the experiments, correcting and critically improvising the manuscript. All authors have read and approved of the final manuscript.
CONFLICT OF INTEREST
The authors declare that they hold no competing interests.
REFERENCES
Ahmed, R., Haq, M., and Chun, B.-S. 2019. Characterization of marine derived collagen extracted from the by-products of bigeye tuna (Thunnus obesus). International Journal of Biological Macromolecules. 135: 668-676.
Ahmed, T., Sun, X., and Udenigwe, C. C. 2022. Role of structural properties of bioactive peptides in their stability during simulated gastrointestinal digestion: A systematic review. Trends in Food Science and Technology. 120: 265-273.
Akita, M., Nishikawa, Y., Shigenobu, Y., Ambe, D., Morita, T., Morioka, K., and Adachi, K. 2020. Correlation of proline, hydroxyproline and serine content, denaturation temperature and circular dichroism analysis of type I collagen with the physiological temperature of Marine teleosts. Food Chemistry. 329: 126775.
Ali, A.M.M., Bavisetty, S.C.B., Prodpran, T., and Benjakul, S. 2019. Squalene from fish livers extracted by ultrasound‐assisted direct in situ saponification: Purification and molecular characteristics. Journal of the American Oil Chemists' Society. 96(9): 1059-1071.
Ali, A.M.M., Benjakul S., and Kishimura H. 2017. Molecular characteristics of acid and pepsin soluble collagens from the scales of golden carp (Probarbus jullieni). Emirates Journal of Food and Agriculture. 450-457.
Ali, A.M.M., Benjakul, S., Prodpran, T., and Kishimura, H. 2018. Extraction and characterisation of collagen from the skin of golden carp (Probarbus jullieni), a processing by-product. Waste and Biomass Valorization. 9: 783-791.
Ali, A.M.M., Kishimura, H., and Benjakul, S. 2018. Extraction efficiency and characteristics of acid and pepsin soluble collagens from the skin of golden carp (Probarbus jullieni) as affected by ultrasonication. Process Biochemistry. 66: 237-244.
AOAC. 2017. Offcial methods of analysis association of official analytical chemists.
Atef, M., Ojagh, S.M., Latifi, A.M., Esmaeili, M., and Udenigwe, C.C. 2020. Biochemical and structural characterization of sturgeon fish skin collagen (Huso huso). Journal of Food Biochemistry. 44(8): e13256.
Benjakul, S., Ali, A. M. M., and Singh, A. 2019. Application of ultrasonication in seafood processing. In Innovative Technologies in Seafood Processing (pp. 131-154). CRC Press.
Chen J., Wang G., and Li Y. 2021. Preparation and characterization of thermally stable collagens from the scales of lizardfish (Synodus macrops). Marine Drugs. 19(11): 597.
Chuaychan, S., Benjakul, S., and Kishimura, H. 2015. Characteristics of acid-and pepsin-soluble collagens from scale of seabass (Lates calcarifer). LWT-Food Science and Technology. 63(1): 71-76.
Gao, L.-L., Wang, Z.-Y., Zheng, L., Zhang, C.-X, and Zhang, D.-Q. 2018. The characterization of acid and pepsin soluble collagen from ovine bones (Ujumuqin sheep). Journal of Integrative Agriculture. 17(3): 704-711.
Gao, Q. 2021. Ultrasonic extraction and identification of carp scale collagen. Journal of Physics: Conference Series (1732(1): 012111. IOP Publishing.
Hong, H., Fan, H., Chalamaiah, M., and Wu, J. 2019. Preparation of low-molecular-weight, collagen hydrolysates (peptides): Current progress, challenges, and future perspectives. Food Chemistry. 301: 125222.
Huang, C. Y., Kuo, J. M., Wu, S. J., and Tsai, H. T. 2016. Isolation and characterization of fish scale collagen from tilapia (Oreochromis sp.) by a novel extrusion–hydro-extraction process. Food Chemistry. 190, 997-1006.
Indriani, S., Benjakul, S., Kishimura, H., Karnjanapratum, S., and Nalinanon, S. 2022. Impact of extraction condition on the yield and molecular characteristics of collagen from asian bullfrog (Rana tigerina) skin. LWT-Food Science and Technology. 162: 113439.
Kameshwar Sharma, Y. V. R., Srivastava, A., Bansal, D., & Srivastava, A. 2024. Fish collagen: Extraction, properties, and prospects. Engineered Biomaterials: Progress and Prospects. 369-419.
Li, J., Wang, M., Qiao, Y., Tian, Y., Liu, J., Qin, S., and Wu, W. 2018. Extraction and characterization of type i collagen from skin of tilapia (Oreochromis niloticus) and its potential application in biomedical scaffold material for tissue engineering. Process Biochemistry. 74: 156-163.
Liu, H., Li, D., and Guo, S. 2007. Studies on collagen from the skin of channel catfish (Ictalurus punctaus). Food Chemistry. 101(2): 621-625.
Ma, M., Ma, L., Yu, W., Zhang, X., Shen, Y., and Zhang, Y. 2018. Research on rapid gelatinization of rabbit skin collagen as effect of acid treatment. Food Hydrocolloids. 77: 945-951.
Nguyen, B., Nguyen, X., Nguyen, K., and Kha, T. 2020. Optimization of treatment conditions for non-collagen removal from yellowfin tuna skin (Thunnus albacares). Chiang Mai University Journal of Natural Sciences. 19(3): 548-562.
Petcharat T., Benjakul S., Karnjanapratum S., and Nalinanon S. 2021. Ultrasound‐assisted extraction of collagen from clown featherback (Chitala ornata) skin: Yield and molecular characteristics. Journal of the Science of Food and Agriculture. 101(2): 648-658. https://doi.org/ 10.1002/jsfa.10677.
Pingret, D., Fabiano-Tixier, A.-S., and Chemat, F. 2013. Ultrasound-assisted extraction. Natural product extraction: Principles and applications. 21: 89.
Pozzolini, M., Scarfì, S., and Giovine, M. 2020. Marine collagen and its biotechnological applications. Encyclopedia of Marine Biotechnology. 2: 1007-1030.
Pongsetkul, J., Benjakul, S., Sumpavapol, P., Vongkamjan, K., and Osako, K. 2018a. Quality of Kapi, salted shrimp paste of Thailand, inoculated with Bacillus spp. K-C3. Journal of Aquatic Food Product Technology. 27(7): 830-843.
Pongsetkul, J., Benjakul, S., Sumpavapol, P., Vongkamjan, K., and Osako, K. 2018. Bacillus subtilis K‐C3 isolated from Thai salted shrimp paste (Kapi): Its extracellular enzymes and use as a starter culture in Kapi production. Journal of Food Biochemistry. 42(6): e12649.
Prommajak, T. and Raviyan, P. 2010. Optimization of gelatin extraction from Thai fish panga (Pangasius bocourti Sauvage) skin. Chiang Mai University Journal of Natural Sciences. 9: 255-272.
Sengkhamparn, N., Lasunon, P., and Tettawong, P. 2019. Effect of ultrasound assisted extraction and acid type extractant on pectin from industrial tomato waste. Chiang Mai University Journal of Natural Sciences. 18(2): 214-225.
Shaik, M.I., Chong, J.Y., and Sarbon, N.M. 2021. Effect of ultrasound-assisted extraction on the extractability and physicochemical properties of acid and pepsin soluble collagen derived from sharpnose stingray (Dasyatis zugei) skin. Biocatalysis and Agricultural Biotechnology. 38: 102218.
Shen, L., Pang, S., Zhong, M., Sun, Y., Qayum, A., Liu, Y., Rashid, A., Baoguo Xu, B., Liang, Q., Ma, H. et al. 2023. A comprehensive review of ultrasonic assisted extraction (UAE) for bioactive components: Principles, advantages, equipment, and combined technologies. Ultrasonics Sonochemistry. 101: 106646.
Sun, L., Hou, H., Li, B., and Zhang, Y. 2017. Characterization of acid-and pepsin-soluble collagen extracted from the skin of Nile tilapia (Oreochromis niloticus). International Journal of Biological Macromolecules. 99: 8-14.
Tan, Y. and Chang, S.K. 2018. Isolation and characterization of collagen extracted from channel catfish (Ictalurus punctatus) skin. Food Chemistry. 242: 147-155.
Wei, P., Zheng, H., Shi, Z., Li, D., and Xiang, Y. 2019. Isolation and characterization of acid-soluble collagen and pepsin-soluble collagen from the skin of hybrid sturgeon. Journal of Wuhan University of Technology- Materials Science Edition. 34(4): 950-959.
Wu, J., Kong, L., Zhang, J., and Chen, W. 2019. Extraction and properties of acid-soluble collagen and pepsin-soluble collagen from silver carp (Hypophthalmichthys molitrix) scales: Prerequisite information for fishery processing waste reuse. Polish Journal of Environmental Studies. 28(4): 2923-2930.
Zou, Y., Wang, L., Cai, P., Li, P., Zhang, M., Sun, Z., Sun, C., Xu, W., and Wang, D. 2017. Effect of ultrasound assisted extraction on the physicochemical and functional properties of collagen from soft-shelled turtle calipash. International Journal of Biological Macromolecules. 105: 1602-1610.
OPEN access freely available online
Natural and Life Sciences Communications
Chiang Mai University, Thailand. https://cmuj.cmu.ac.th
Sri Charan Bindu Bavisetty1, Supatra Karnjanapratum2, *, Jaydeep Dave1, Daniel Tua Purba1, Tanaji Kudre3, Wahyu Haryati Maser4, Nur Maiyah1, Passakorn Kingwascharapong5, and Ali Muhammed Moula Ali1,*
1 School of Food-Industry, King Mongkut’s Institute of Technology Ladkrabang, Bangkok 10520, Thailand.
2 Division of Marine Product Technology, Faculty of Agro-Industry, Chiang Mai University, Chiang Mai 50100, Thailand.
3 Department of Meat and Marine Sciences, Central Food Technological Research Institute, Mysore, Karnataka 570020, India.
4 Department of Food Technology, Faculty of Agriculture, Universitas Sumatera Utara, Medan 20155, Indonesia.
5 Department of Fishery Products, Faculty of Fisheries, Kasetsart University, Bangkok 10900, Thailand.
Corresponding author: Ali Muhammed Moula Ali, E-mail: ali-muhammed.mo@kmitl.ac.th,
Supatra Karnjanapratum E-mail: supatra.ka@cmu.ac.th
Total Article Views
Editor: Sirasit Srinuanpan,
Chiang Mai University, Thailand
Article history:
Received: September 14, 2023;
Revised: November 9, 2023;
Accepted: November 23, 2023;
Online First: December 13, 2023

