
Antifungal Effect and Durability of Chitosan Oligosaccharide Coating on Heat-cured Polymethylmethacrylate Surface
Pattaraporn Buanpech, Pisaisit Chaijareenont, Phenphichar Wanachantararak, Patcharawan Silthampitag*Published Date : December 12, 2023
DOI : https://doi.org/10.12982/NLSC.2024.004
Journal Issues : Number 1, January-March 2024
Abstract This study aimed to investigate the antifungal effect against Candida albicans (ATCC® 10231) and the durability of chitosan oligosaccharide (COS) coated on heat-polymerized polymethylmethacrylate (PMMA). The various concentrations of COS solution, 0.5, 1 and 2-fold of minimum fungicidal concentration (0.5, 1 and 2 MFC) were prepared and applied to PMMA specimens treated with sandblasting (SB), application of 3-aminopropyltriethoxysilane (APS), and a combination of both procedures (SB&APS). The toothbrush abrasion test was done to evaluate the remaining COS (n=10). The antifungal effect was determined through the colony count method (n=3). The number of fungal colonies was recorded at 16 and 48 hours. Data were analyzed using two-way and one-way ANOVA at the 95% confidence level. The result of the toothbrush abrasion test revealed that COS concentration and surface treatment affected coating durability. APS and SB&APS groups showed a high percentage of COS and significantly differed from SB groups (P = 0.00). For the antifungal effect, coatings with 1 MFC and 2 MFC demonstrated no difference, but coating with 0.5 MFC differed from others (P = 0.00). At 16 and 48 hours, PMMA coated with 1 MFC and 2 MFC by APS and SB&APS methods inhibited C.albicans. COS can be coated onto PMMA using APS and can inhibit C.albicans. With further development, this method could be applied to treat denture stomatitis alternatively to conventional antifungal drugs.
Keywords: Candida albicans, Chitosan, Oligochitosan, Polymethylmethacrylate, Silanes
Citation: Buanpech, P., Chaijareenont, P., Wanachantararak, P., Silthampitag, P. 2024. Antifungal effect and durability of chitosan oligosaccharide coating on heat-cured polymethylmethacrylate surface. Natural and Life Sciences Communications. 23(1): e2024004.
INTRODUCTION
Chitosan is a kind of biopolymer containing an amino-polysaccharide structure and is produced by the deacetylation of chitin, which is presented in crab shells and insects. The amino group, the primary and secondary hydroxyl groups, and the acetamide group are functional groups of chitosan (Figure 1). The amino contents are the main factors contributing to differences in their structures and physiochemical properties, which make it easy to generate intra- and inter-molecular hydrogen bonds (Zhang et al., 2010; Zhou et al., 2021). The molecular weight and degree of deacetylation of chitosan affect its properties. Even though it possesses several advantages, the primary drawback is its insoluble nature in water. Thus, several studies have been conducted on the improvement of the chitosan solubility (Sakai et al., 2001; Fu and Xiao, 2017; Xuan Du and Xuan Vuong, 2019).
Chitosan oligosaccharide (COS) is a chitosan oligomer formed by hydrolysis. Due to the shorter chain length of COS, the free amino group is exposed to a greater extent than in conventional chitosan. As a result, COS has improved solubility and is easy to apply for therapeutic purposes. Additionally, low molecular weight COS (molecular weight less than 1,500 Dalton) is soluble throughout a broad pH range (Naveed et al., 2019). Previous studies also indicated that COS solution might be applied as an antifungal agent, including against Candida albicans (C.albicans) (Je and Kim, 2012; Peña et al., 2013; Phil et al., 2018).
Several studies investigated the combination of chitosan and its derivatives with polymethylmethacrylate (PMMA), the most common denture base material. The mixture of chitosan and PMMA was intended to inhibit Candida spp (Song et al., 2016; Chander and Venkatraman, 2022). However, the studies on the mechanical properties of this mixture were controversial. Several studies have described coating the denture base with various materials, such as titanium dioxide (Obata et al., 2017) and co-polymer containing sulfobetaine methacrylamide (Hirasawa et al., 2018), to reduce microbial adhesion to the denture surface. In addition, Zuo et al reported that denture base resins with organic-inorganic hybrid coatings exhibited greater color stability than uncoated resins. This suggests that these coatings have the potential to prevent or reduce pigment changes that can occur over time (Zuo et al., 2016). However, the coating method for prevention is complex and difficult to implement in clinical settings. Therefore, the present investigation was intended to concentrate on the treatment of denture stomatitis.
There was a study about coating chitosan on PMMA surfaces to decrease fungal infection. The study aimed to develop a novel approach for adhering chitosan to PMMA surface using silica-coated alumina sandblasting (Wieckiewicz et al., 2016). The previous methods required additional processes, including neutralization after coating specimens with acetic chitosan solution. However, no studies have discussed coating PMMA surfaces with COS regarding techniques or antifungal effects against C.albicans.
Silane coupling agents are organosilicon compounds with two functionally reactive groups. There are various types of silane coupling agents available: this study, selected silane coupling agents based on evidence of common application and solubility parameters. To achieve a strong bonding, the solubility parameter of the adhesive and the adherent should be comparable (Chaijareenont et al., 2012). 3-aminopropyltriethoxysilane (APS), an amino silane, was one of the most commonly mentioned silane coupling agents in dentistry literature. APS has similar solubility properties to MMA and has been applied cooperatively with chitosan and PMMA Fields (Chen et al., 2007; Martin et al., 2007; Vlachopoulou et al., 2008; Xie et al., 2010). In addition, APS terminates with a primary amine group (Figure 2) that can be chemically modified by a linker molecule or connected to various materials, including proteins and biopolymers (Martin et al., 2007).

Figure 1. Structure of chitosan oligosaccharide (COS) with the functional group, -NH2 and -OH.

Figure 2. Structure of 3-aminopropyltriethoxysilane (APS) with a 3-aminopropyl and three ethoxy silane groups.
Coating chitosan on polymethylmethacrylate (PMMA) surface was mentioned in previous studies (Wieckiewicz et al., 2016). However, research has yet to be conducted to demonstrate the coating of COS on a heat-cured PMMA specimen that resembled a realistic removable denture. Therefore, this study aims to determine the antifungal effect of heat-cured PMMA coated with various concentrations of COS against C.albicans and to examine the durability of COS coating. The null hypothesis is that there are no differences in the antifungal effect and durability of COS-coated PMMA, regardless of the surface treatment method or COS concentration.
MATERIALS AND METHODS
Experimental design
According to the objectives of this study, two distinct tests were conducted. The sample size was determined using the G* Power program (Version 3.1.9.6) based on data obtained from the pilot study. In the first test, ninety samples were divided into nine groups (n = 10) to reflect various surface treatment techniques and COS coating concentrations. The second test was the antifungal effect of coated specimens against C.albicans, with nine specimens divided into three groups (n = 3) with varying concentrations of COS coating. This second test continued the first, in which the most effective surface treatment procedure was chosen to evaluate the antifungal effect. The materials used in this study were revealed in Table 1.
Table 1. Lists of materials utilized in this experiment.
|
Material |
Trade name |
Manufacturer |
Lot no. |
|
Heat-cured PMMA |
VERTEX™ Rapid Simplified |
Vertex-Dental B.V., UTRECHT, Netherlands |
WX162P08 |
|
MMA Monomer |
VERTEX™ Rapid Simplified |
Vertex-Dental B.V., UTRECHT, Netherlands |
WW252L02 |
|
Chitosan Oligosaccharide |
Marine Bio Resources: Chitosan Oligosaccharide (COSF) |
Marine Bio Resources Co., Ltd, Samutsakorn, Thailand |
22154/1-1 |
|
Sabouraud dextrose broth |
HIMEDIA™ Sabouraud dextrose broth (SDB) |
HiMedia Laboratories.Pvt.Ltd., Mumbai, India |
0000516873 |
|
Sabouraud dextrose agar |
HIMEDIA™ Sabouraud dextrose agar (SDA) |
HiMedia Laboratories.Pvt.Ltd., Mumbai, India |
0000525968 |
|
Silane coupling agent |
TCI 3-aminopropyltriethoxysilane |
Tokyo Chemical Industry Co., Ltd., Tokyo, Japan |
STY6M-CF |
Specimens preparation
Flat-cylindrical specimens were fabricated using heat-cured PMMA (Vertex Rapid Simplified, Vertex-Dental B.V., UTRECHT, Netherlands) by compression molding technique following ISO 20795-1:2013(E) and ADA standard No.12. The finishing and polishing of each specimen were achieved by the sequential steps detailed in Table 2. The thickness of 2 mm and the diameter of 15 mm were confirmed with a digital vernier caliper (CD15-AX, Mitutoyo Co. Ltd., Kanagawa, Japan). Each specimen’s surface roughness (Ra) of each specimen was measured using a profilometer (SJ-310, Mitutoyo Co. Ltd., Kanagawa, Japan). Three measurements were made for each specimen, and the mean value was analyzed as Ra (µm). One person recorded all measurements. All specimens had a mean surface roughness of 0.9274 ± 0.0946 µm. Then, The specimen was cleaned by an ultrasonic cleaner (Easyclean, Renfert GmbH Company, Hilzingen, Germany) at a water temperature of 50 °C for 3 minutes (Charasseangpaisarn et al., 2016) before being immersed in sterile water at a temperature of 37 °C for 48 hours to allow residual methylmethacrylate (MMA) monomer to be released (Vallittu et al., 1995; Wonglamsam et al., 2016).
Table 2. Description of the finishing and polishing procedures performed before coating.
|
Steps |
Tool used |
Details |
Duration |
|
Coarse finishing |
Acrylic finishing bur (Tungsten carbide, coarse) |
8000 RPM; Round uniform movements with light pressure |
3-4 minutes |
|
Fine finishing |
Smoothening bur (Tungsten carbide, fine) |
8000 RPM; Round uniform movements with light pressure |
3-4 minutes |
|
Polishing |
Silicone rubber bur (Medium grain) |
4000 RPM; Round uniform movements with light pressure |
3-4 minutes |
|
|
Waterproof Silicon carbide abrasive paper (2000-grits) |
Placed one side of each specimen toward abrasive paper and moved specimen side to side (range 20 mm from starting point) under running water |
5 times |
Prepared the inoculum of C. albicans and determined the minimum fungicidal concentration (MFC)
C.albicans (ATCC® 10231) isolates were grown on 2% Sabouraud dextrose broth (SDB) (HIMEDIA™, HiMedia Laboratories. PVt. Ltd., Mumbai, India) for 24 hours at 37 °C in the incubator (Memmert BE400, Memmert GmbH + Co.KG, Schwabach, Germany). The fungal suspension's optical density (OD) was adjusted close to 0.5 McFarland standard at 530 nm and measured with a spectrophotometer (Beckman DU650, Beckman Coulter Inc., California, USA). This suspension approximately contained C.albicans 1 x 107 CFU/ml, which was confirmed by serial dilution, spread plate technique, and direct colony count. COS solution was created by diluting 1 g of COS powder in 1 ml of sterile water. The solution was homogenized by an electric mixer (VTX 3000L, Dominique Dutscher SAS, Bernolsheim, France). The evaluation of minimum inhibitory concentration (MIC) was done by pipetting 50 µl of the fungal suspension, which approximately contained C.albicans 50,000 cells, and dropping it into microcentrifuge tubes (1.5 ml) with various volumes of COS solution (50 µl, 100 µl, 150 µl, 200 µl, 250 µl, 300 µl, 350 µl, 400 µl, and 450 µl). The contents in the tubes were homogenized and cultured for 24 hours at 37 °C in the incubator. The streak plate technique was continued to examine the MFC. The MFC of COS was calculated and revealed that COS solution at a concentration of 830 mg/ml could eradicate C.albicans. After that, each concentration’s inhibition percentage was evaluated by serial dilution and spread plate technique on Sabouraud dextrose agar (SDA) (HIMEDIA™, HiMedia Laboratories. Pvt. Ltd., Mumbai, India).
Coating procedure
The steps of the coating are shown in Figure 3. Three different PMMA surface treatment techniques were used; sandblasting (SB), APS application (APS), and a combination of the two techniques (SB&APS). For the SB and SB&APS groups, the surface treatment of each specimen was started with sandblasting. One flat side of the specimen surface was treated with aluminum oxide particles with the size of 110 µm, using a sandblasting machine (Renfert GmbH Company, Kitzingen, Germany) at 2.8 bar pressure and 30 mm from the specimen surface (Wieckiewicz et al., 2016) for 30 seconds. Blasted specimens were rinsed for 20 seconds with distilled water to remove loose particles before being wiped dry. The surface of the sandblasted specimens was observed under the scanning electron microscope (SEM) and element energy dispersive x-ray spectrometer (EDS) (JSM-IT500HR, InTouchScope™, JEOL Ltd., Tokyo, Japan). For the APS and SB&APS groups, the mixture of 70% v/v Ethanol and APS (TCI, Tokyo Chemical Industry Co. Ltd., Tokyo, Japan) was prepared to obtain a 2% v/v APS solution. Dropped 50 µl of the 2% v/v APS solution on one flat surface of each specimen and spread it to cover the entire surface with a disposable micro brush. The coated specimens were dried for 20 seconds with the warm-air drying at 60 ± 5 °C using a hairdryer (Dyson™, Malmesbury, UK)(Kay Khine et al., 2021), resulting in the formation of a silanized layer on the PMMA surface (Vlachopoulou et al., 2008). COS solutions with concentrations of 415 (0.5 MFC), 830 (1 MFC), and 1,660 mg/ml (2 MFC) were prepared. 300 µl of the solution was dropped on the center of each specimen. The specimens were subjected to a drying process in a hot air oven set at a temperature of 55°C for 120 minutes (Wieckiewicz et al., 2016).
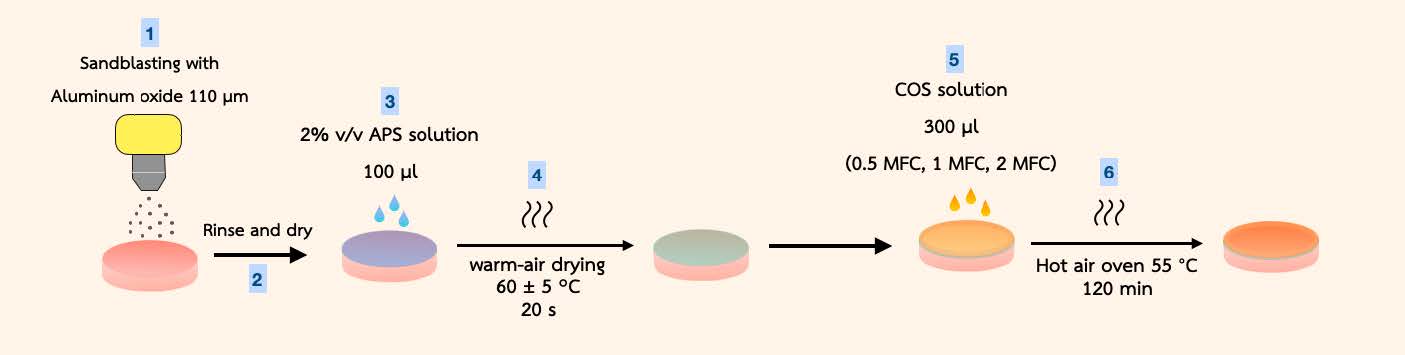
Figure 3. The illustration shows the coating procedures for the PMMA specimens used in this study. Steps 1, 4, 5, and 6 were for SB groups. For the APS group, steps 3, 4, 5, and 6 were completed. While SB&APS groups were prepared according to steps 1 to 6.
Toothbrush abrasion test of COS-coated PMMA surface
This experiment required the construction of a custom-made brushing apparatus. Five electric toothbrushes (Oral-B PRO 2 2000, Procter & Gamble, Bangkok, Thailand) were positioned with brush heads (Oral-B EB602, Procter & Gamble, Bangkok, Thailand). The brushing force was controlled by a stainless steel calibration weight of 1 N (OIML F2) positioned on top of each brush head (Wiegand et al., 2013; Pujarern et al., 2021). The toothbrushes were locked in place on a flat surface and adjusted until a long axis of the bristles was perpendicular to the specimen surface. The specimens were mounted in aluminum wells with Polyvinyl siloxane putty (3M™ Express™ XT Putty, Minnesota, USA) and secured the well on a flat surface. Before conducting the toothbrush abrasion test, the picture of the coating area was recorded to be the control by a full-frame Digital-Single-Lens-Reflex (DSLR) camera (Canon EOS RP, Canon Inc., Tokyo, Japan) using a macro lens (Canon EF 100mm f/2.8L Macro IS USM, Canon Inc., Tokyo, Japan) at the fixed distance 15 cm from specimens. The toothbrush abrasion test was performed without toothpaste in oscillated and rotated at 8,800 rounds per minute for 30 seconds to simulate 7-day wear from brushing (Santos et al., 2013). The battery was fully charged before testing for each group and each brush head was replaced after finishing the test for one group. The camera was utilized to record the surface of the coated specimens. The ImageJ Program version 1.53 was used to measure the area of COS coating on each specimen both before and after the toothbrush abrasion test. Subsequently, the measured values were converted into the percentage of remaining COS.
Antifungal effect of COS-coated PMMA specimen
According to the abrasion test results, the effective PMMA surface treatment for coating COS was chosen for antifungal effect evaluation. Consequently, the specimens were divided into seven groups (n = 3) based on the COS coating concentration and surface treatment method: APS (1 MFC), APS (2 MFC), SB+APS (0.5 MFC), SB+APS (1 MFC) and SB+APS (2 MFC), as well as the positive control group (uncoated PMMA with SDB) and the negative control group (uncoated PMMA with fungal suspension). Ethylene oxide gas was used to disinfect the specimens before testing. Then, each specimen was placed on the flat bottom of a 24-well cell culture plate (well diameter 15.1 mm). For the coated specimens and negative control group, 100 µl of C.albicans suspensions (100-fold dilution of 0.5 McFarland standard suspension which contained 10,000 cells of C.albicans) was inoculated into each well and incubated at 37 °C for 90 minutes to settle the yeasts to the specimen. After incubation, 1 ml of SDB was added to each well, followed by 16 hours of incubation at 37 °C. Suspension (100 µl) was diluted with the serial dilution technique, and C.albicans colony in the suspension was quantified using the spread plate method at a level of detection within 500 CFU per plate. Independent tests with three repetitions were undertaken, and the entire procedure was repeated at 48 hours.
Statistical analysis
Data were analyzed using IBM® SPSS® Statistics version 26 (SPSS Inc., Chicago, IL, USA). The normality of all data was confirmed using the Shapiro-Wilk normality test. Two-way and one-way analysis of variance (ANOVA) and post hoc tests were used for comparing data. A P-value less than 0.05 was considered statistically significant.
RESULTS
Morphology of the specimens
After sandblasting with aluminum oxide particles (110 µm), the blasted PMMA specimen showed more scratch and porosity than the non-blasted specimen under SEM (Figure 4). The EDS analysis revealed the different surface compositions of the specimens, reported in %weight. Non-blasted specimens contained 61.2% carbon and 38.8% oxygen. Sandblasted specimens exhibited 7.39% aluminum, 55.73% carbon, and 36.87% oxygen (Figure 5).

Figure 4. SEM pictures of heat-cured PMMA surface at the 100x magnification A. non-blasted PMMA and B. PMMA surface blasted with aluminum oxide particles at 2.8 bar pressure.
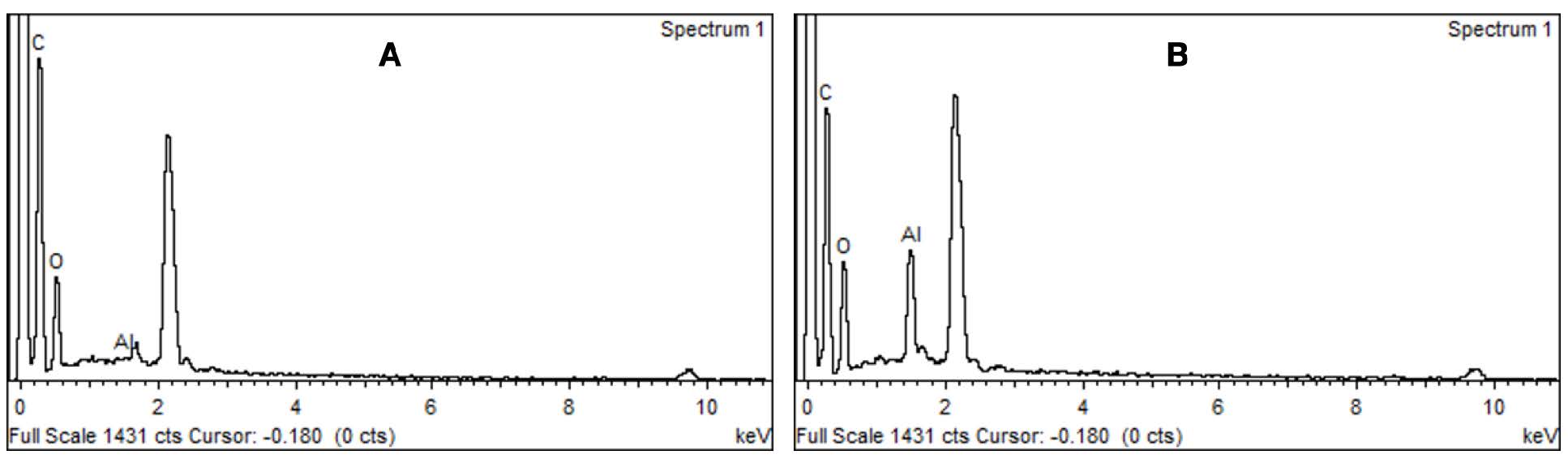
Figure 5. The EDS analysis of heat-cured PMMA surface. A. non-blasted PMMA and B. PMMA surface blasted with aluminum oxide particles at 2.8 bar pressure.
Toothbrush abrasion
The percentage of remaining COS was influenced by surface treatment methods and the concentration of COS solution. The SB+APS groups had the largest area of remaining COS, followed by the APS groups, while the SB groups had the least area of COS left (Table 3). In the APS and SB+APS groups, 1 MFC and 2 MFC revealed statistically significantly higher percentages of COS than 0.5 MFC. The SB group showed no significant differences in the percentage of remaining COS between concentrations. (Figure 6-7)

Figure 6. Bar graphs show the mean percentage of COS remaining for each group after the toothbrush abrasion test for 30 seconds. Each group (a, b, c, d and e) had statistically significant differences using Dunnett’s T3 post hoc tests at a 95% confidence level. The surface treatment and concentration of COS affect the mean percentage of remaining COS in APS and SB+APS groups, in which 1 MFC and 2 MFC show more percent than 0.5 MFC (P<0.001).

Figure 7. After a toothbrush abrasion test for 30 seconds, the PMMA specimen coated with COS by different surface treatment methods shows a variable amount of remaining COS.; A. Before brushing (Control), B. Sandblasting, C. APS and D. Sandblasting combined with APS.
Table 3. The result of the toothbrush abrasion test on heat-cured PMMA specimen presented in mean percentage of remaining COS (%).
|
Group |
|
|
|
|
Surface treatment |
COS concentration (mg/ml) |
n |
Mean (SD) |
|
SB |
415 (0.5 MFC) |
10 |
32.275 (4.997) |
|
|
830 (1 MFC) |
10 |
33.267 (4.036) |
|
|
1,660 (2 MFC) |
10 |
32.335 (4.171) |
|
APS |
415 (0.5 MFC) |
10 |
61.182 (2.567) |
|
|
830 (1 MFC) |
10 |
69.077 (0.974) |
|
|
1,660 (2 MFC) |
10 |
68.457 (1.684) |
|
SB + APS |
415 (0.5 MFC) |
10 |
71.301 (0.960) |
|
|
830 (1 MFC) |
10 |
76.093 (1.693) |
|
|
1,660 (2 MFC) |
10 |
75.438 (1.512) |
Antifungal effect
The concentration affected the percentage of inhibition of COS solution. 830 mg/ml was the lowest concentration that completely inhibited C.albicans growth and was considered as the MFC in this study (Figure 8-9).
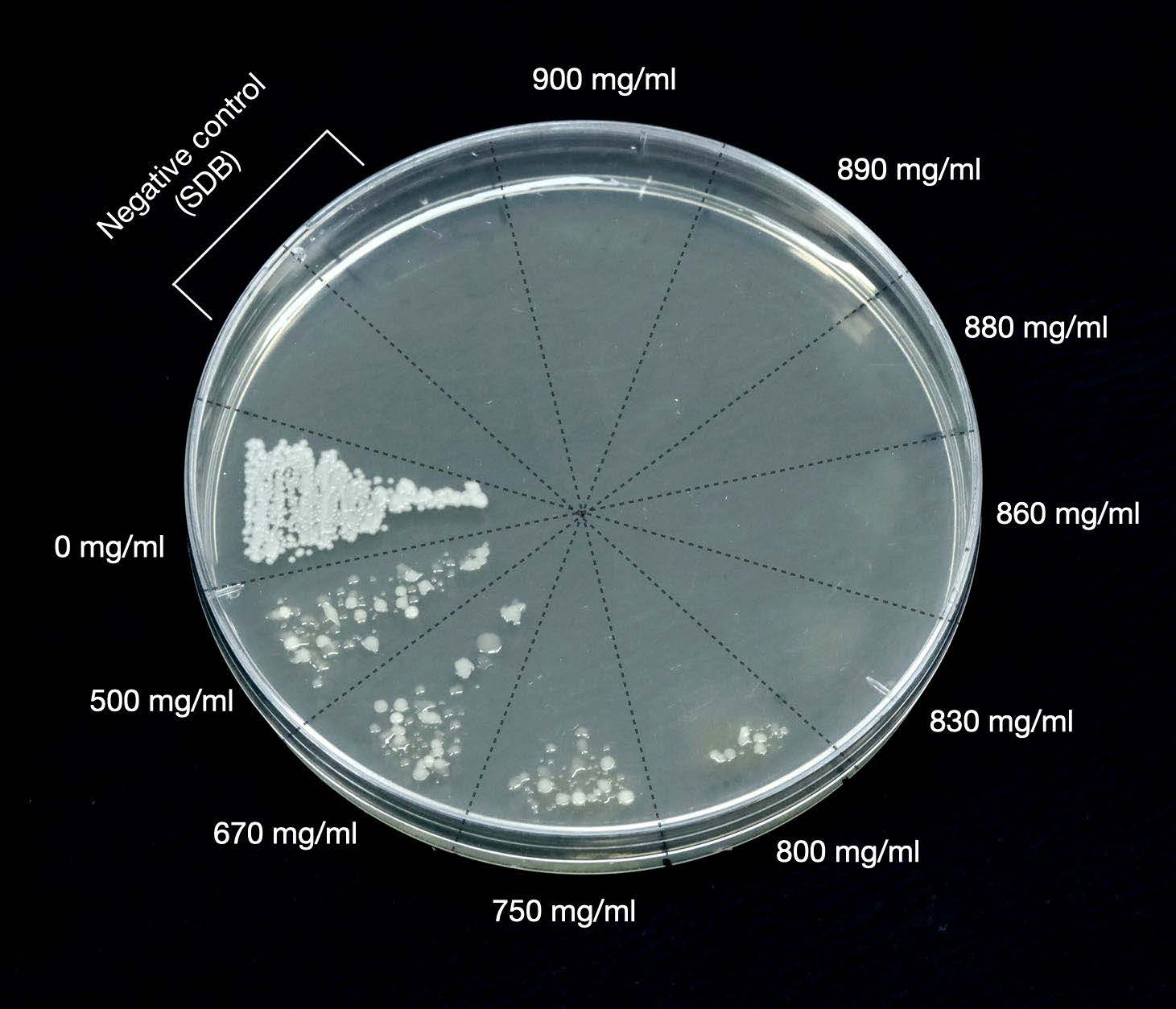
Figure 8. The streak plate technique was performed to evaluate the MFC of COS solution. The complete inhibition started at the concentration of 830 mg/ml.
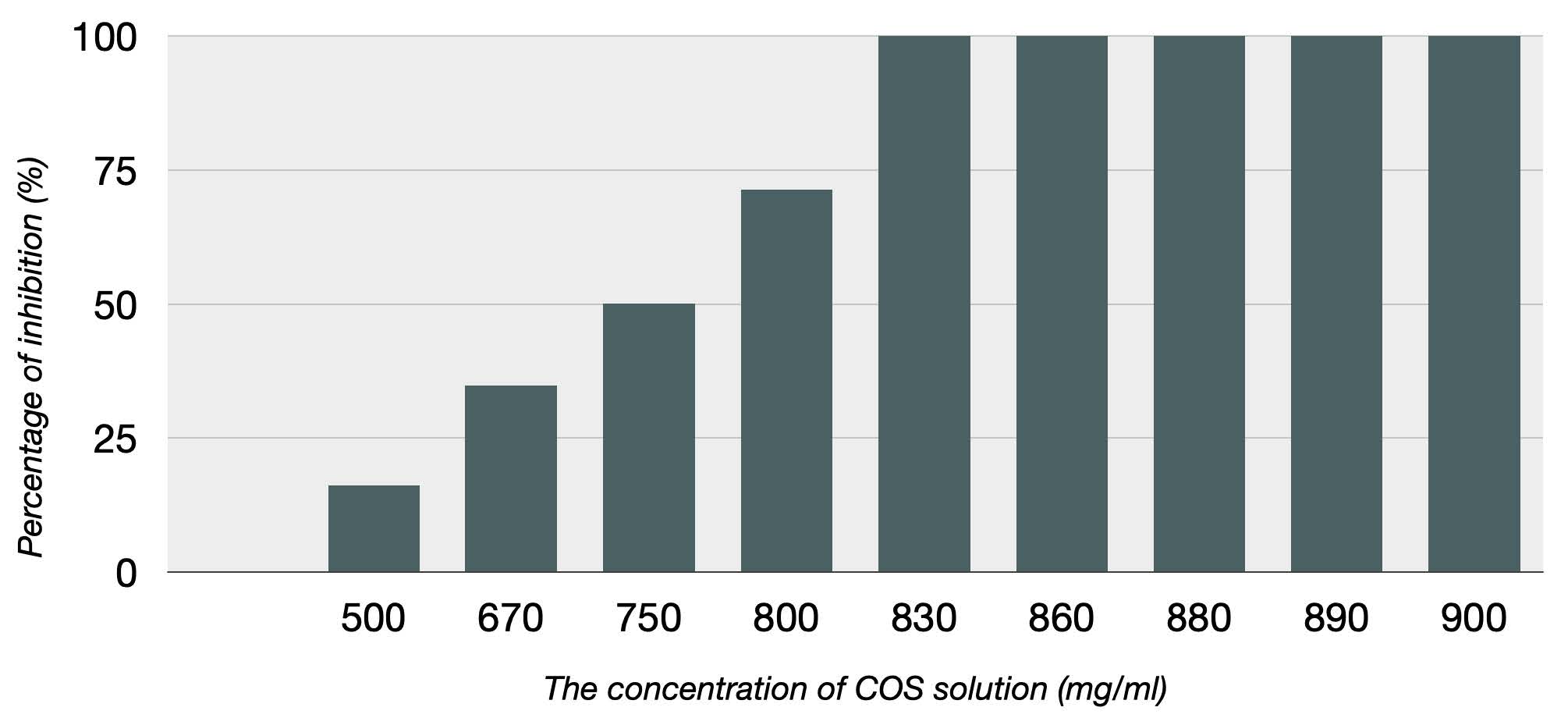
Figure 9. The bar graphs show the percentage of C.albicans inhibited by COS solution. 500 mg/ml, 670 mg/ml, 750 mg/ml, and 800 mg/ml inhibited 16.13%, 34.74 %, 50.12%, and 71.29%, respectively. Complete inhibition (100%) was observed between 830 and 900 mg/ml.
For the coated PMMA, the complete inhibition of C.albicans was found in specimens coated with 1 MFC and 2 MFC concentration by both APS and SB+APS treatment methods, and a statistically significant difference between the SB+APS group at 0.5 MFC and the non-coated group (control) at both 16 and 48 hours. (Figure 10)

Figure 10. The mean colony count of C.albicans is shown as a bar graph at 16 and 48 hours. The fungal colony was observed in the negative control group (uncoated PMMA) and the PMMA coated with COS at 0.5 MFC by SB&APS.
DISCUSSION
The present study evaluated the antifungal effect of COS coated on the PMMA surface against C.albicans and its durability using the toothbrush abrasion test. According to the results, the null hypothesis is that there is no significant difference in durability and antifungal effect of various surface treatment methods and concentrations, was rejected. The surface treatment methods influenced the bonding between PMMA and COS. Sandblasting with aluminum oxide particles did not affect the adhesion of the two substrates. However, the previous study mentioned the silica-coated alumina particles to promote the adhesion between chitosan and PMMA. The silicate molecule in the particle can create covalent and ionic bonds with functional groups of chitosan, especially the amino group (Wieckiewicz et al., 2016). Aluminum oxide particles were employed in this investigation, therefore there was no influence from silicate molecules. While applying APS improved the percentage of remaining COS on the PMMA surface. Combining sandblasting with APS gave the highest percentage of remaining COS on PMMA.
Figure 11 illustrates the chemical connection between PMMA APS and COS. The possible interaction between APS and COS might be described as the amine group of APS can interact with the hydroxyl group of COS via hydrogen bonds. In addition, APS may form strong intra- and inter-molecular hydrogen bonds with its amino group, also possessing a strong affinity towards the hydroxyl groups of COS. These interactions may result in the development of a cage-like structure. Thermoplastic materials with a hydrocarbon backbone, such as PMMA, were reported to not react with the amino group of amino silanes, including APS (Xie et al., 2010). A higher percentage of remaining COS on PMMA discs in the group using APS could be due to the increase of surface energy after applying the silane on the PMMA surface (Lung and Matinlinna, 2012). APS might facilitate the diffusion of COS solution on the surface of PMMA or through the porosity of PMMA, as well as establish micromechanical locking between COS and PMMA. After the toothbrush abrasion test, the sandblasting with alumina oxide and APS produced a substantially more significant proportion of COS coating. Sandblasting can induce microabrasions on the acrylic surface, increasing the surface area and causing interlocking between the coupling agent, chitosan, and PMMA. In addition, the impeded alumina particle on PMMA during grit blasting might have generated =Al-O-Si bonds during the silanization (Lung and Matinlinna, 2012; Visuttiwattanakorn et al., 2017). However, increasing the surface roughness of PMMA can increase the severity of fungal infection since surface roughness is one of the factors affecting adherence to C.albicans. Therefore, the risk-benefit balance must be assessed before implementing this method.

Figure 11. The possible interaction between sandblasted PMMA, APS and COS.
According to this investigation, the surface treatment method did not affect the growth of C.albicans, and the antifungal effect of PMMA coated with COS at 1 MFC and 2 MFC concentrations was not different after 16 and 48 hours. Denture wearers were advised to remove their dentures at night while sleeping, resulting in a total wear time of approximately 16 hours. While 48 hours of testing were based on the time that C.albicans was at the highest virulent growth phase, the stationary phase (Westwater et al., 2005). The mechanism of fungicide was not investigated in this work. However, previous studies mentioned that chitosan is a cationic polysaccharide that reacts with the negative charge on the fungal cell wall and harms the target cells by increasing permeability and finally leading to cell leakage and death (Park et al., 2008; Soliman et al., 2015; Phil et al., 2018). COS was investigated for its ability to affect C.albicans cells via this mechanism. COS exhibits the ability to interact with the fungal cell membrane, leading to the efflux of potassium ions (K+), acidification of the extracellular environment, elevation of the transmembrane potential, and enhanced absorption of calcium ions (Ca2+). Consequently, these processes collectively contribute to a reduction in the negative charge present on the surface of the fungal cell, ultimately resulting in the inhibition of the growth of C.albicans (Peña et al., 2013). Chitosan also prevents the formation of fungal infections by limiting the creation and maturation of biofilm and C.albicans adhesion to human mucosal cells. Furthermore, chitosan can penetrate the nucleus of fungal cells and bind to DNA, inhibiting the creation of mRNA and protein (Kong et al., 2010). Due to the limitations of this study, which included the use of chitosan oligosaccharide obtained from a single source, future studies must compare various sources of chitosan or other different derivatives to use COS-coated on PMMA surfaces for the treatment of oral candidiasis. Furthermore, the stability of COS coating should be investigated further to generate an efficient antifungal agent that can persist on PMMA longer and be more convenient for the patient to use. According to previous studies, chitosan has mucoadhesive properties and was used as a carrier for drugs in the ophthalmologic treatment (Khangtragool et al., 2008; 2009). Therefore, combining COS with other antifungal drugs, such as nystatin, should be investigated further to produce an effective antifungal effect. Moreover, chitosan should be used with caution in individuals who are allergic to crustaceans since the studies on allergic symptoms caused by chitosan are controversial (Kato et al., 2005; Waibel et al., 2011).
CONCLUSION
Within the limitations of this study, it can be concluded that the adhesion between PMMA and COS cannot be primarily attributed to micromechanical retention achieved through sandblasting. By using APS, COS can be coated onto PMMA. COS solution with a concentration of 830 mg/ml, when coated on PMMA, has been found to impact the activity of C. albicans. This suggests that it could potentially serve as an alternative to standard antifungal medicines, which are known to elicit drug resistance and cause changes in taste perception. This study may lead to the investigation of alternative treatments for patients with Candida-associated denture stomatitis, particularly those who require the use of their dentures for any purpose during pathology treatment.
ACKNOWLEDGMENTS
The authors would like to express sincere gratitude to Thanapat Sastraruji, Ph.D. (Dental Research Center, Faculty of Dentistry, Chiang Mai University) for the statistical analysis advice. This study was supported by a research grant from the Faculty of Dentistry, Chiang Mai University.
CONFLICT OF INTEREST
The authors report no conflict of interest.
REFERENCES
Chaijareenont, P., Takahashi, H., Nishiyama, N., and Arksornnukit, M. 2012. Effects of silane coupling agents and solutions of different polarity on PMMA bonding to alumina. Dental Materials Journal. 31: 610-616.
Chander, N. G. and Venkatraman, J. 2022. Mechanical properties and surface roughness of chitosan reinforced heat polymerized denture base resin. Journal of Prosthodontic Research. 66: 101-108.
Charasseangpaisarn, T., Wiwatwarrapan, C., and Leklerssiriwong, N. 2016. Ultrasonic cleaning reduces the residual monomer in acrylic resins. Journal of Dental Sciences. 11: 443-448.
Chen, J. H., Liu, Q. L., Zhang, X. H., and Zhang, Q. G. 2007. Pervaporation and characterization of chitosan membranes cross-linked by 3-aminopropyltriethoxysilane. Journal of Membrane Science. 292: 125-132.
Fu, Y. and Xiao, C. 2017. A facile physical approach to make chitosan soluble in acid-free water. International Journal of Biological Macromolecules. 103: 575-580.
Hirasawa, M., Tsutsumi-Arai, C., Takakusaki, K., Oya, T., Fueki, K. and Wakabayashi, N. 2018. Superhydrophilic co-polymer coatings on denture surfaces reduce Candida albicans adhesion—An in vitro study. Archives of Oral Biology. 87: 143-150.
Je, J.Y. and Kim, S.K. 2012. Chitooligosaccharides as potential nutraceuticals: Production and bioactivities. Advances in Food and Nutrition Research. 65: 321-336.
Kato, Y., Yagami, A., and Matsunaga, K. 2005. A case of anaphylaxis caused by the health food chitosan. Japanese Journal of Allergology. 54: 1427-1429.
Kay Khine, P. P., Tichy, A., Abdou, A., Hosaka, K., Sumi, Y., Tagami, J., and Nakajima, M. 2021. Influence of silane pretreatment and warm air-drying on long-term composite adaptation to lithium disilicate ceramic. Crystals. 11: 86.
Khangtragool, A., Ausayakhun, S., Leesawat, P., Molloy, R. and Laokul, C. 2008. Stability of chitosan solutions for potential use in ocular drug delivery. Chiang Mai University Journal of Natural Sciences. 7: 209-217.
Khangtragool, A., Ausayakhun, S., Leesawat, P., Molloy, R. and Laokul, C. 2009. Evaluation of the use of chitosan in ocular drug delivery of vancomycin. Chiang Mai University Journal of Natural Sciences. 8: 1-9.
Kong, M., Chen, X. G., Xing, K., and Park, H. J. 2010. Antimicrobial properties of chitosan and mode of action: A state of the art review. International Journal of Food Microbiology. 144: 51-63.
Lung, C. Y. K. and Matinlinna, J. P., 2012. Aspects of silane coupling agents and surface conditioning in dentistry: An overview. Dental Materials. 28: 467-477.
Martin, H. J., Schulz, K. H., Bumgardner, J. D., and Walters, K. B. 2007. XPS study on the use of 3-aminopropyltriethoxysilane to bond chitosan to a titanium surface. Langmuir. 23: 6645-6651.
Naveed, M., Phil, L., Sohail, M., Hasnat, M., Baig, M. M. F. A., Ihsan, A. U., Shumzaid, M., Kakar, M. U., Khan, T. M., and Akabar, M. 2019. Chitosan oligosaccharide (COS): An overview. International Journal of Biological Macromolecules. 129: 827-843.
Obata, T., Ueda, T. and Sakurai, K. 2017. Inhibition of denture plaque by TiO2 coating on denture base resins in the mouth. The Journal of Prosthetic Dentistry. 118: 759-764.
Park, Y.K., Kim, M.H., Park, S.C., Cheong, H.S., Jang, M.K., Nah, J.W., and Hahm, K.S. 2008. Investigation of the antifungal activity and mechanism of action of LMWS-chitosan. Journal of microbiology and biotechnology. 18: 1729-1734.
Peña, A., Sánchez, N. S., and Calahorra, M. 2013. Effects of chitosan on Candida albicans: Conditions for its antifungal activity. BioMed research International. 2013: 527549
Phil, L., Naveed, M., Mohammad, I. S., Bo, L., and Bin, D. 2018. Chitooligosaccharide: An evaluation of physicochemical and biological properties with the proposition for determination of thermal degradation products. Biomedicine and Pharmacotherapy. 102: 438-451.
Pujarern, P., Rodanant, P., Warinsiriruk, E., and Rattanasuwan, K. 2021. Evaluation of the optimum brushing force on dental plaque removal: An in vitro study. Mahidol Dental Journal. 41: 75-82.
Sakai, Y., Hayano, K., Yoshioka, H., and Yoshioka, H. 2001. A novel method of dissolving chitosan in water for industrial application. Polymer Journal. 33: 640-642.
Santos, M., Soo, S., and Petridis, H. 2013. The effect of Parylene coating on the surface roughness of PMMA after brushing. Journal of Dentistry. 41: 802-808.
Soliman, A. M., Fahmy, S. R., and Mohamed, W. A. 2015. Therapeutic efficacy of chitosan against invasive candidiasis in mice. The Journal of Basic & Applied Zoology. 72: 163-172.
Song, R., Zhong, Z., and Lin, L. 2016. Evaluation of chitosan quaternary ammonium salt-modified resin denture base material. International Journal of Biological Macromolecules. 85: 102-110.
Vallittu, P. K., Miettinen, V., and Alakuijala, P. 1995. Residual monomer content and its release into water from denture base materials. Dental Materials. 11: 338-342.
Visuttiwattanakorn, P., Suputtamongkol, K., Angkoonsit, D., Kaewthong, S., and Charoonanan, P. 2017. Microtensile bond strength of repaired indirect resin composite. The Journal of Advanced Prosthodontics. 9: 38-44.
Vlachopoulou, M., Tserepi, A., Pavli, P., Argitis, P., Sanopoulou, M., and Misiakos, K. 2008. A low temperature surface modification assisted method for bonding plastic substrates. Journal of Micromechanics and Microengineering. 19: 015007.
Waibel, K. H., Haney, B., Moore, M., Whisman, B., and Gomez, R. 2011. Safety of chitosan bandages in shellfish allergic patients. Military Medicine. 176: 1153-1156.
Westwater, C., Balish, E., and Schofield, D. A. 2005. Candida albicans-conditioned medium protects yeast cells from oxidative stress: A possible link between quorum sensing and oxidative stress resistance. Eukaryotic Cell. 4: 1654-1661.
Wieckiewicz, M., Wolf, E., Richter, G., Meissner, H., and Boening, K. 2016. New concept of polymethyl methacrylate (PMMA) and polyethylene terephthalate (PET) surface coating by chitosan. Polymers. 8: 132.
Wiegand, A., Burkhard, J. P. M., Eggmann, F., and Attin, T. 2013. Brushing force of manual and sonic toothbrushes affects dental hard tissue abrasion. Clinical Oral Investigations. 17: 815-822.
Wonglamsam, A., Kaewkornpradit, W., Nagaviroj, N., and Kanchanavasita, W. 2016. Effect of processing and curing procedures on residual monomer levels of denture base materials. Mahidol Dental Journal. 38: 305-311.
Xie, Y., Hill, C. A., Xiao, Z., Militz, H., and Mai, C. 2010. Silane coupling agents used for natural fiber/polymer composites: A review. Composites Part A: Applied Science and Manufacturing. 41: 806-819.
Xuan Du, D. and Xuan Vuong, B. 2019. Study on preparation of water-soluble chitosan with varying molecular weights and its antioxidant activity. Advances in Materials Science and Engineering. 2019: 8781013.
Zhang, J., Xia, W., Liu, P., Cheng, Q., Tahirou, T., Gu, W., and Li, B. 2010. Chitosan modification and pharmaceutical/biomedical applications. Marine Drugs. 8: 1962-1987.
Zhou, J., Wen, B., Xie, H., Zhang, C., Bai, Y., Cao, H., Che, Q., Guo, J., and Su, Z. 2021. Advances in the preparation and assessment of the biological activities of chitosan oligosaccharides with different structural characteristics. Food and Function. 12: 926-951.
Zuo, W., Feng, D., Song, A., Gong, H., and Zhu, S. 2016. Effects of organic-inorganic hybrid coating on the color stability of denture base resins. The Journal of Prosthetic Dentistry. 115: 103-108.
OPEN access freely available online
Natural and Life Sciences Communications
Chiang Mai University, Thailand. https://cmuj.cmu.ac.th
Pattaraporn Buanpech1, Pisaisit Chaijareenont1, Phenphichar Wanachantararak2, Patcharawan Silthampitag1, *
1 Department of Prosthodontics, Faculty of Dentistry, Chiang Mai University, Chiang Mai 50200, Thailand.
2 Dental Research Center, Faculty of Dentistry, Chiang Mai University, Chiang Mai 50200, Thailand
Corresponding author: Patcharawan Silthampitag, E-mail: patcharawan.sil@cmu.ac.th
Total Article Views
Editor: Supon Ananta
Chiang Mai University, Thailand
Article history:
Received: April 10, 2023;
Revised: November 13, 2023;
Accepted: November 23, 2023;
Online First: December 12, 2023

