
Modification of Lakra (Pakistani) Coal by Chemicals Washing and Heat Treatment and Its Application for Removal of Organic Acids (Prop Ionic Acid and Chloroacetic Acid) from Aqueous Solution. F. Akbar Jan*, Naimat Ullah, and Shahzad Hameed
Published Date : 2019-08-23
DOI : https://doi.org/10.12982/CMUJNS.2019.0023
Journal Issues :
Number 3 , July-September 2019
ABSTRACT
In the present study, Lakra coal was heat treated and washed with different chemicals (KMnO4 (0.5M), NaOH (0.1M), HNO3 (0.1M), HCl (0.1M), and H2O2 (0.1M)) and investigated as adsorbent for the removal of organic acid pollutants such as propionic and chloroacetic acids from aqueous solution. The results revealed that virgin coal despite of evacuating for generating porosity the adsorption of both acids near to nil. Pre-treatment of coal samples with a variety of chemicals increased the adsorption capabilities as compared to virgin coal. KMnO4, HCl and HNO3 treated samples showed maximum adsorption among all the samples. Prominent pores generation was observed in the treated samples shown by SEM images of virgin and variously treated coal samples. The aforementioned chemicals also reduced ash, volatile matter, sulphur and ash contents of the coal. At 210 minutes maximum adsorption capacities for chemicals modified coal samples were observed and above no appreciable change was found. The results showed that kinetics of sorption properties of organic acids fitted well with Lagergern pseudo second order model with high correlation coefficiency (r2) for modified coal samples. For the adsorption of both propionic acid and chloroacetic acid Langmuir model best described the adsorption process. It is concluded that only heat treatment of the coal cannot increase the adsorption capacity for effective adsorption pre-treatment with either of the chemical would be more effective.
Keywords: Organic pollutants acids, Chemicals treated coal, Adsorption, Kinetic study
INTRODUCTION
Pollution of the environment caused by industrial wastewater is a common problem faced by many countries. Due to awareness of the environmental deterioration wastewater treatment has gained considerable attention over the years. A number of pollutants including inorganic and organic i.e. propionic and chloroacitic acids are added to the sewerage water by various industries, causing serious water pollution. These acids are not only dangerous for human life but also have fatal effects on aquatic life. For strict environmental compliance the chemical industries globally facing challenges of wastes disposal though some industries do have conventional wastewater treatment plants. However, most of the organic pollutants cannot be removed by conventional wastewater treatment because of their stability towards light as well as to aerobic digestion. Adsorption has been found to be a superior technique for organic pollutants control (Ayranci and Duman, 2006; Liu et al., 2009; López-Velandia et al., 2014; Cheng et al., 2017).
Keeping in view the adverse health hazards associated with organic acids like propionic and chloroacetic acids wastewater must be processed for retention of these acids before such water is added to fresh water streams (Li et al., 2013). Like for strong mineral acids like hydrochloric acid these organic acids should be treated with similar care (Bada and Potgieter-Vermaak, 2008).
Organic compounds present in industrial wastewater are toxic, non- biodegradable and difficult to be removed by biological treatment processes. In order to meet strictly with environmental regulations, the technologies like solvent extraction, chemical oxidation and activated carbon adsorption has been applied to remove toxic and non biodegradable organic compounds from portable as well as domestic and industrial wastewater supplies. Amongst the candidate retention methods, activated carbon adsorption has been proved effective for organics removal from water supplies (Sun et al., 2018; Zhang et al., 2018). Energy efficient, clean, responsive to market demands and intrinsically safe are basic requirements of a chemical process. Zero waste chemicals generation, use of less hazardous chemicals, low energy consumption, recyclable materials are now a days the aims of chemical industries and they are driving towards more environmentally friendly processes. Development of different means, techniques, processes and methods for the removal of these pollutants is the challenge for scientist. Adsorption being a surface phenomenon depends on narrow particle size distribution, specific surface area, and porosity of an adsorbent (Sun et al., 2010; Ugurlu and Karaoglu, 2011; Hussein et al., 2016; Sun et al., 2018). This technology is most widely accepted to remove organic pollutants from effluent owing to its simple operational steps, low cost – effectiveness and high efficiency. Beneficiation techniques are used for the increase of adsorption rate and capacity of adsorbents using various chemicals like HCl, NaOH as reported by many researchers. These chemicals not only increase the surface area but also enhance surface morphology thereby increasing the adsorption capacity (Nadiye-Tebbiruka and Lungani, 2018). Adsorption capacity linearly increases with increase in specific surface area, carbon content and finer particle size of adsorbent. Chemical treatment method has been found to improve the adsorption capability of carbonaceous materials (Nethaji et al., 2013). Pakistan has the world largest coal reserves that is the ‘Thar’ coal deposits ‘Lakra’ coal deposits etc.
The aim of the present study was to investigate Lakra coal as adsorbent for the removal of organic pollutant acids (propionic acid and chloroacetic acid) after heating at high temperature for generation emptiness and channels. Then to modify the heat treated coal with some chemicals like KMnO4, NaOH, HNO3, HCl and H2O2 for increasing the adsorption capacities. Kinetics models and Langmuir sorption isotherm application was also the purpose of the study.
MATERIAL AND METHODS
Sample collection
The coal sample was collected through PMDC (Pakistan Mineral Development Corporation) according to the standard methods of sample collection from Lakra coalmine. Run of mine (ROM) coal was obtained in the form of lumps. It was crushed and then it was sieved through 212-µm-mesh screen. This coal was stored for further study in a clean petri dish so that dust may not enter.
Washing of coal with chemicals
Raw coal was washed with 1M HCl, 1M HNO3, 1M H2O2, 1M NaOH, and 0.5 M KMnO4 solutions. For each chemical used for washing, 50 g sample was soaked in a volume of 200 ml of solution in a round bottom flask at specified temperature for 3 h under continuous stirring. The coal slurry was filtered and then washed the coal residue with hot deionized water untill free from chemicals used indicated by using different indicators i.e. using AgNO3 as an indicator for chlorine.
Physicochemical characterization of coal
The proximate ASTM 3286, ASTM 3175, ASTM 3174, ASTM 3173 methods were used for finding the volatile matter, ash content and moisture content of coal.
Determination of Total Sulfur in Coal
Total sulfur contents were determined according to the standard Escha mixture method. The percentage of sulfur was calculated using formula:
Sulfur (% by weight) = 13.74 (A - B + 0.008)/w
A = weight of BaSO4 in grams found in coal B
= weight of BaSO4 in grams found in Blank W = weight of test sample
Determination of Total Chlorine in Coal
Total chlorine contents were also calculated using Escha mixture method Total chlorine percentage was calculated using formula
Chlorine (% by weight) = (b - a) 3.546 F/w
Where, b = volume of standard potassium thiocyanate solution in the blank
a = volume of standard potassium thiocyanate solution in the virgin coal.
F = normality of potassium thiocyanate
W = weight of coal sample.
Heat treatment of coal
About 60-70g of fine coal (212µm size) sample was weighted in a ceramic boat and was then put in quartz tube of the tubular furnace. The contents were then heated at the rate of 10 K / min to the temperature of 600oC for 4 hours. Argon was delivered into the mullite tube at a flow rate of 1.L/ min for ensuring inert atmosphere during whole process. The sample was cooled down to room temperature (Nadiye-Tabbituka and Lungani, 2018).
Adsorption of propionic acid and chloroacetic acid on virgin and variously treated coal samples
The propionic acid and chloroactic acid were prepared by dissolving the analytical reagent in deionized water. One gram adsorbent and 50 ml of adsorbate solution were added to the solutions in 100 ml flasks mounted on a constant temperature (25°C) water bath shaker at 120 rpm to keep the adsorbent suspended. The mixture was shacked for different intervals of time in order to optimize the time. All samples were filtered. The filtrate was titrated against standard (0.1N) KOH solution. The amount of acid adsorbed was calculated by formula
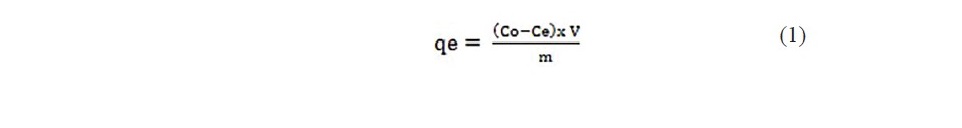
where Co is the initial organic pollutants concentration, (mg/lit) Ce is the final organic pollutants concentration, concentration (mg/lit),V is the volume of organic pollutants concentration, taken for adsorption study and m is the weight of adsorbent in grams. Similarly the leached coal samples were studied for adsorption of prop ionic acid and chloroacitic acids (Dina et al., 2012; Nethaji et al., 2013).
Kinetic experiments
The kinetics of adsorption of Propionic acid and Chloroacetic acid was studied by applying Lagergan pseudo first order equation and 2nd order models (equation a and b). The kinetic experiments were performed separately using different initial acid concentrations like 2, 10, 15, 25, 35 and 45 mg/L. The amount adsorbed at time t was calculated by
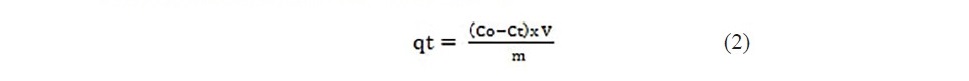
where Co and Ct (mg/L) are the concentrations at initial time and time t, respectively (Zhang et al., 2014).
Origin 7.0 software was used for Langmuir isotherm parameters by fitting the data with linear regression analysis.
RESULTS
Physiochemical parameters of Lakra coal
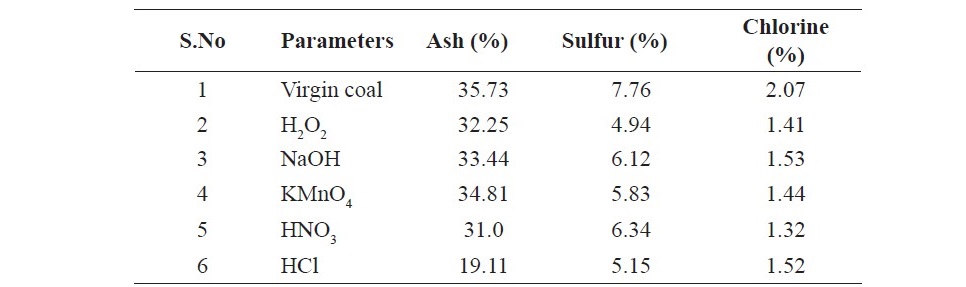
The data given in Table 1 it shows the physicochemical parameters of coal. Moisture, volatile matter, ash and fixed carbon contents were 5.82, 19.62, 35.73 and 34.74% respectively. From the data in Table 2 it can be seen that 0.1M HCl has caused maximum extrusion of mineral matter from the coal. The ash contents in HCl treated coal sample was found to be 19.11%. The ash contents was 31.00%, 34.81% , 33.44% and 32.25% in HNO3, NaOH and H2O2 treated coal samples. It was found that H2O2 has effectively removed sulfur (4.94%) from coal followed by HCl (5.12%) compared to other chemicals.
Table 1. Physicochemical properties of Lakra coal.
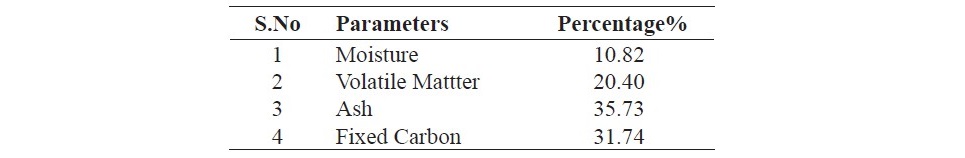
Table 2. Percentage of ash, sulfur and chlorine contents of virgin and various treated samples.
Adsorption kinetics of propionic acid and chloroacetic acid onto variously treated coal samples
The effect of contact time on the adsorption of propionic acid on treated coal samples at 298 K is presented in Figure 1 and 2. The SEM images given in Figures 3 (a, b, c, d, e, f) showed that HNO3 and HCl treated coal has high porosity indicated. Large holes can be seen throughout image which indicate the rejection of iron based minerals and CaCO3 and MgCO3 which was effectively removed by these chemicals conferring the increase in amorphous property. The kinetics of adsorption of propionic acid and chloroacetic acid onto various treated coal samples was studied by applying Lagergan or pseudo first order equation and 2nd order models (equation 3 and 4). The plot are given in Figure 4(a, b, c, d, e) and Figures 5(a, b, c, d, e).Table 3 and 4 shows the rate constants for the adsorption of propionic acid and chloroacetic acid onto various treated coal samples.
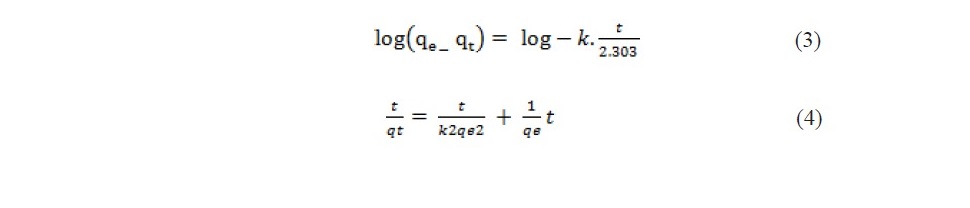
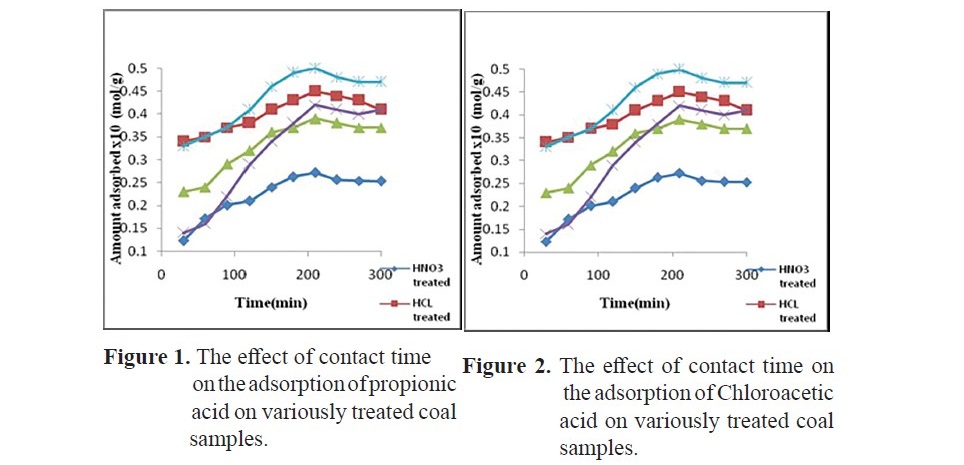

Figure 3. SEM images of raw and treated coal samples.
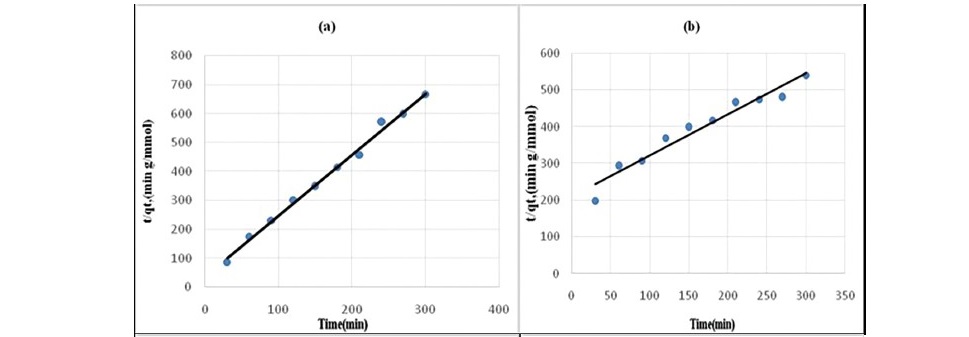
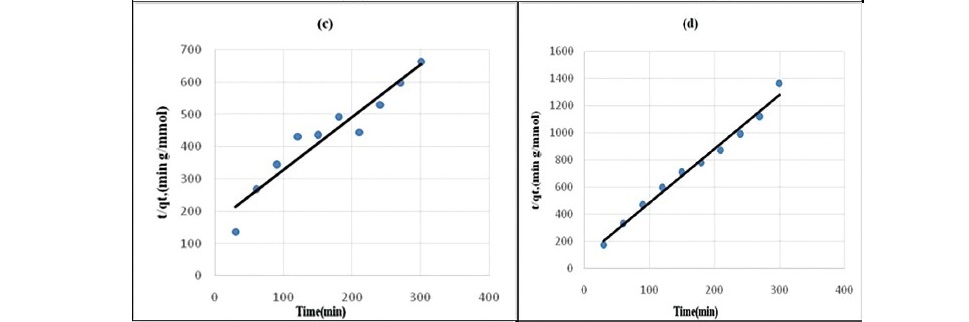
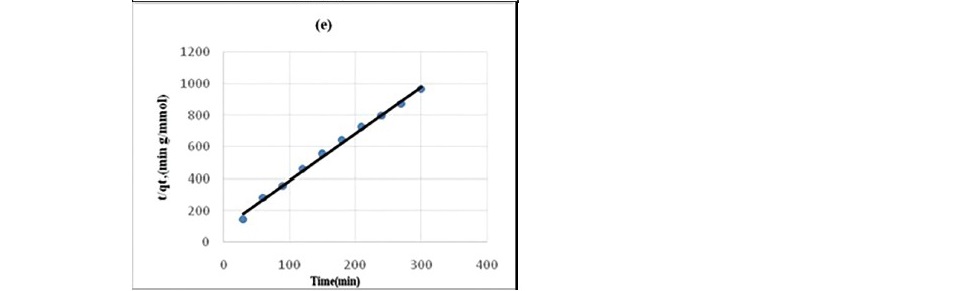
Figure 4. Pseudo second-order kinetics plot for the sorption of Propionic acid
(a) HNO3 treated coal (b) HCl treated coal (c) KMnO4 treated coal (d) H2O2treated coal (e) NaOH treated coal.
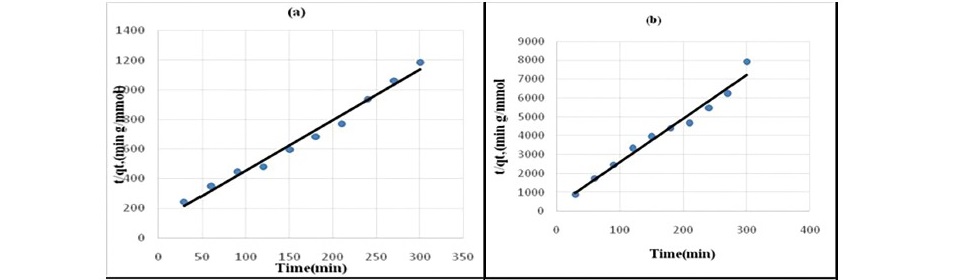
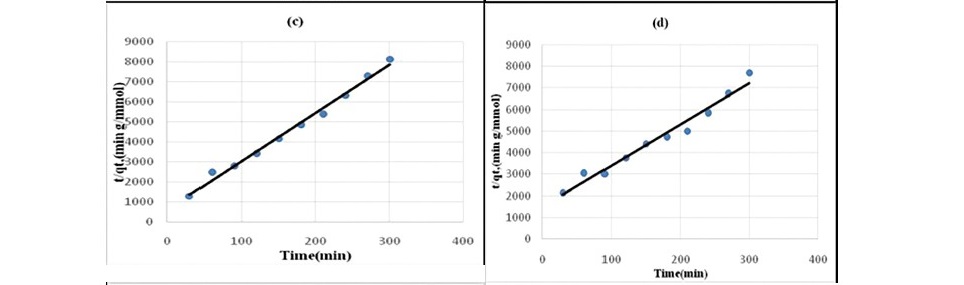
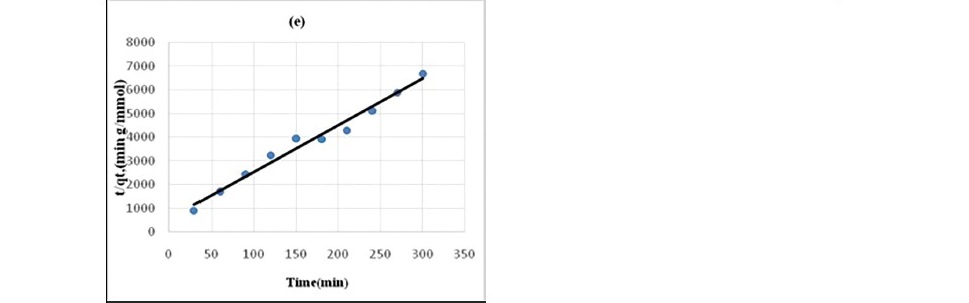
Figure 5. Pseudo second-order kinetics plot for the sorption of chloroacetic acid
(a) HNO3 treated coal (b) HCl treated coal (c) KMnO4 treated coal (d)H2O2 treated coal (e) NaOH treated coal.
Lagergren pseudo-first order model when applied to the kinetic data it cannot fit the model well. For pseudo-second-order the correlation coefficients are also closer to unity (Table 3 and 4) therefore, the sorption can be approximated more appropriately by the pseudo-second-order kinetic model than the first-order kinetic model. The results are similar to reports made on activated carbon (Ho and McKay, 1998). Langmuir isotherm model (equation 5) is used for studying the parameters of the equilibrium state (Ho, 2004).
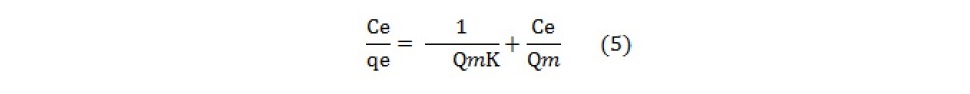
where Qm is the amount of adsorbate at complete monolayer coverage (mg g-1). Figure 6 (a, b, c, d, e) and 7 (a, b, c, d, e) represents the Langmuir sorption isotherms for propionic acid and chloroacetic acid on variously treated samples.
Table 3. Pseudo second-order rate constants by linear method for the sorption of Propionic acid on variously treated coal samples.
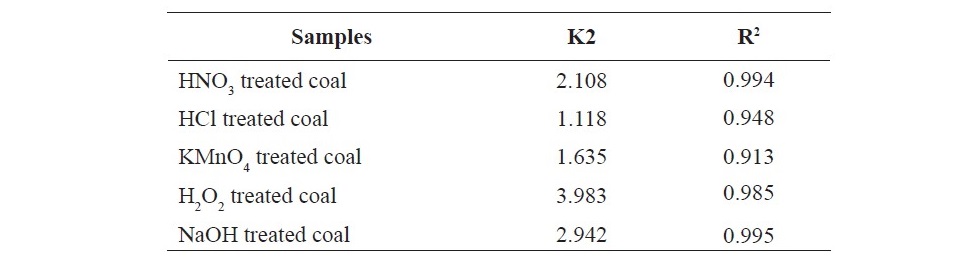
Table 4. Pseudo second-order rate constants by linear method for the sorption chloroacetic acid on variously treated coal samples.
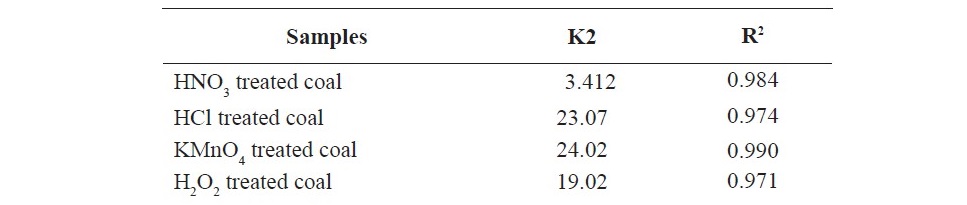
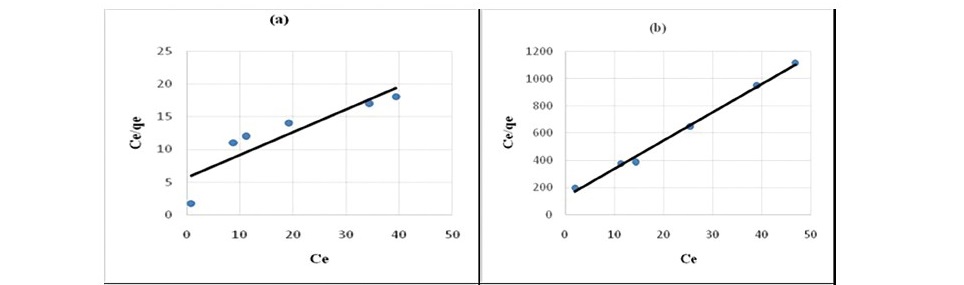
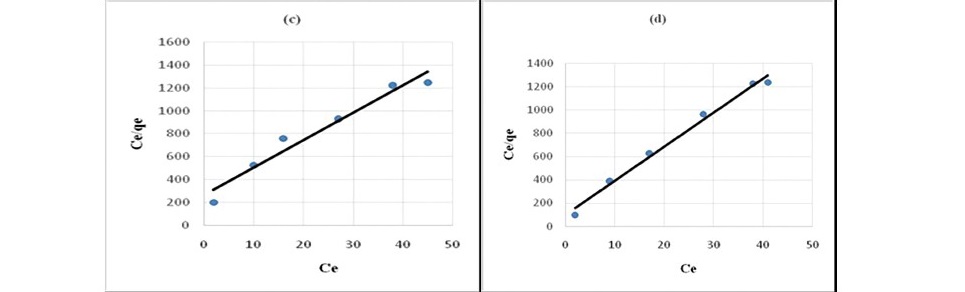
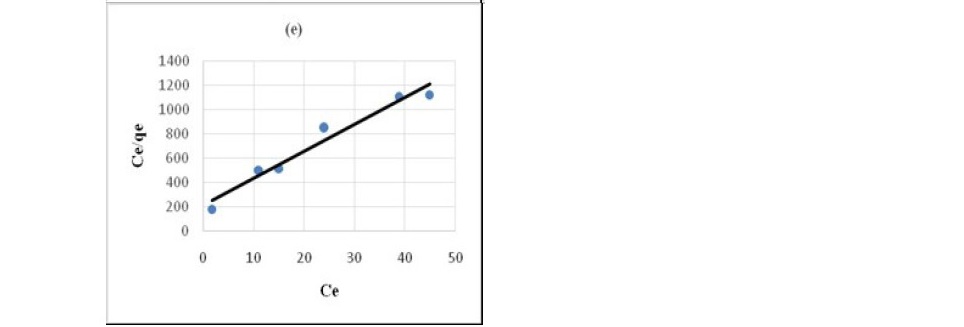
Figure 6. Langmuir plot for sorption of propionic acid (a) HNO3 treated coal (b) HCl treated coal (c) KMnO4 treated coal (d) H2O2 treated coal
(e) NaOH treated coal.
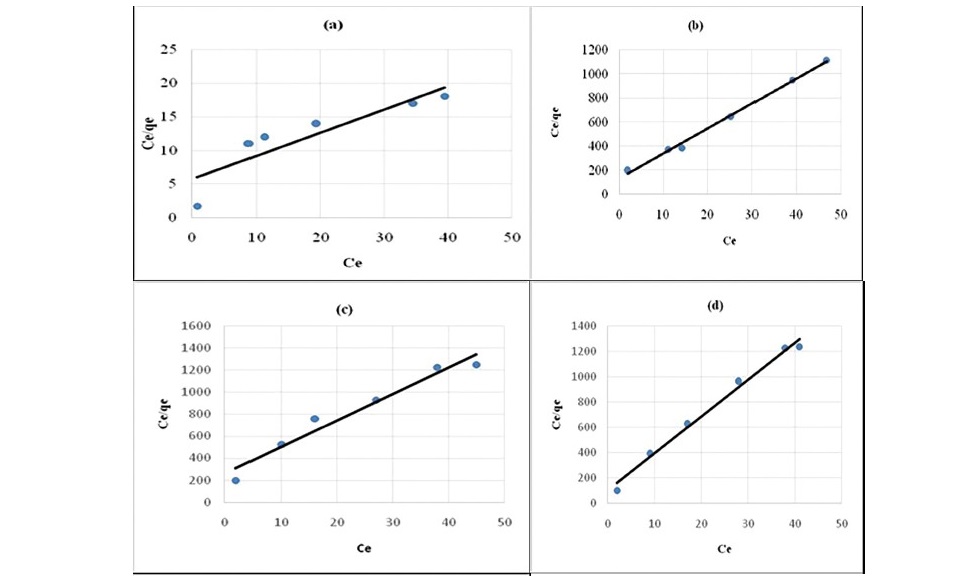
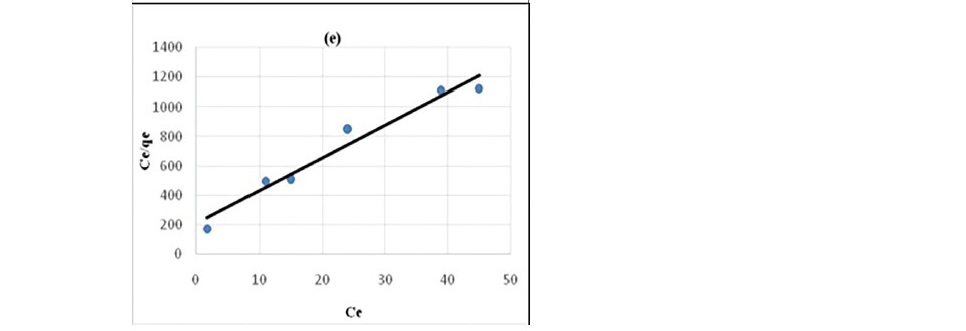
Figure 7. Pseudo Langmuir plot for the sorption of chloroacetic cid (a) HNO3 treated coal (b) HCl treated coal (c) KMnO4 treated coal (d) H2O2 treated coal (e) NaOH treated coal.
DISCUSSION
The fixed carbon and moisture contents of the virgin coal are low while volatile matter and ash content are high. The high ash contents, volatile matter and low fixed carbon indicate that it is low rank coal and also has low calorific values. The inorganic mineral matters is usually associated with the clay particles that are packed into the coal matrix.
The high concentration of sulfur is undesirable in coal. On burning sulfur is converted to sulfur oxides and combines with atmospheric water there by causes acid rain (Ting-chu, 2008; Liu, 2010; Pansuk and Vinitnantharat, 2011; Ken and Nandi, 2018). Besides these the synthetic fuels obtained from high sulfur coal during liquefaction or gasification contain high percentage of sulfur decreasing the fuel quality and also produces fouling with engine system. This can be attributed to the oxidation of the sulfur to soluble compounds by treatment with hydrogen peroxide. Degree of demineralization increases with the removal of mineral matters present in coal. The major mineral matters such as SiO2 and Al2O3 present in the coal dissociates into alkali solution forming alkali silicates and aluminates.
High concentration of chlorine is undesirable because it causes fouling of heating surfaces when burned in boilers and also damage brick work of coke oven and gas retort. At high temperature prevailing during carbonization it suffers volatilization and reacts with the refractoriness forming glaze. It is also found that coals containing more than 15% chlorine may cause trouble in super heaters of boilers that are mechanically fired (Wang et al., 2016; Sharma et al., 2017). All the chemicals effectively caused removal of chlorine contents from the coal. Lower percentage of chlorine was found in HNO3 treated sample that was 1.32% followed by HCl and H2O2 respectively. So HNO3 can be considered best for chlorine removal. The data obtained (Figure 1 and 2) showed that poor adsorption of propionic acid occurred on virgin coal sample. This may be due to the fact that in raw coal there are limited number of sites available for interaction with adsorbent. Since acid washing can results in a reduction on the mineral content of coal and charcoal therefore significant adsorption of both the acids occurred on the treated coal samples. As acids and other chemicals treatment can increase porosity of solids which are applied as adsorbents in the chromatographic packing, purification and to support structures for catalytic processes. The SEM images given in Figures 3 (a, b, c, d, e) shows that the raw coal sample appears to be a relatively less porous material. The micrographs indicates that the external surface of the chemically activated coal is full of cavities. It is clear that coal modified with chemical like hydrochloric acid and nitric acid have more mounds, cracks, scraps and fragments, while the surface of samples modified by sodium hydroxide are relatively smooth. Crushing of coal can destroy their porous structure enhancing surface area but still the original shape of some particles is retained and appeared to have a very porous and ordered network. It can be seen from the graph the presence a less number of pores present (as indicated by black areas) which shows that the coal has less capability of adsorption.
The chemicals used for treatment are good oxidizing agents and has increased the pore size by oxidizing other inclusions with in the coal pores and hence increases the porosity of the coal. The crevices and some grains of various sizes in large holes are also present.
It can be concluded from the SEM micrographs that the porous structure was formed due to evolution of most of the organic volatiles. Adsorption of propionic acid and chloroacetic acid increased on various treated coal samples with increase in time duration. Maximum adsorption capacities for heat and chemicals modified coal samples was observed at 210 minutes above which there almost no further increase in the adsorption. The contact time curves showed that the adsorption capacity increased gradually before 60 min and reached quasi- equilibrium after 210 min, beyond which there was no significant increase in adsorption capacity. The data also shows that adsorption increases with increase in time duration of stirring. The increase in adsorption with respect to time of contact might correspond to multi layer adsorption.
It may be concluded that pseudo second order equation (Figure 3) is best applicable to the explanation of kinetic data and the mechanism adsorption of propionic acid and choloroacetic acid onto variously treated coal sample.
It has been confirmed by various studies that in static system the ratio of mass, volume and initial concentration of solution affect sorption kinetics parameters (Ho, 2006; Sari et al., 2008; Barma et al., 2018). Constant reaction rate was found to be influenced by the initial solution concentration and ratio of sorbent mass to solution volume. For the adsorption of both propionic acid and chloroacetic acid Langmuir model (Figure 6 and 7) best described the adsorption process. This indicated that sorption of organic acids onto chemicals modified coal samples followed a monolayer formation.
CONCLUSION
The Lakra coal in raw form is not an effective sorbent for the removal of organic pollutant acids. Treatment of the coal understudy with a variety of chemicals leads to the washing of mineral matter and other inclusion from the coal thereby increasing the surface area and adsorption capacity. Heating of coal also remove volatile matter from the coal and can increase surface area and active sites in the coal. Treatment of Lakra coal with HNO3 and HCl solutions enhanced the surface morphology and porosity to a greater extent and thus increased the adsorption capacity of the coal under study. HNO3, HCl and H2O2 caused removal of maximum amount of sulfur and chlorine contents from the coal. The kinetics of adsorption of propionic acid and choloroacetic on to treated coal samples was studied using Lagergan pseudo first and second order and Langmuir non linear models. Lagergan pseudo second order model best described the adsorption of propionic acid and choloroacetic on to treated coal samples. The adsorption of chloroacetic acid onto variously treated coal samples follow a monolayer adsorption kinetics.
REFERENCES
Ayranci, E., and Duman, O. 2006. Adsorption of aromatic organic acids onto high area activated carbon cloth in relation to wastewater purification. Journal of Hazardous Materials. 25: 542-52. https://doi.org/10.1016/ j.jhazmat.2005.12.029
Bada, S.O., and Potgieter-Vermaak, S. 2008. Evaluation and treatment of coal fly ash for adsorption application. Leonardo Electronic Journal of Practices and Technologies. 12: 37-48.
Barma, S.D., Sathish, R., Baskey, R.K., and Biswal, S.K. 2018. Chemical beneficiation of high-ash indian noncoking coal by alkali leaching under low-frequency ultrasonication. Energy Fuels. 32(2): 1309–1319. https://doi.org/10.1021/acs.energyfuels.7b03291
Cheng, J., Yin, W., Chang, Z., Lundholm, N., and Jiang, Z. 2017. Biosorption capacity and kinetics of cadmium(II) on live and dead Chlorella vulgaris. Journal of Applied Phycology. 29(1): 211-221. https://doi.org/10.1007/ s10811-016-0916-2
Dina, D.J.D., Ntieche, A.R., Ndi, J.N.K., and Mbadcam, J. 2012. Adsorption of acetic acid onto activated carbons obtained from maize cobs by chemical activation with zinc chloride (ZnCl2). Research Journal of Chemical Sciences. 2(9): 42-49.
Ho, Y.S. 2004. Citation review of Lagergren kinetic rate equation on adsorption reactions. Scientometrics.59(1):171-177.https://doi.org/10.1023/B:SCIE. 0000013305.99473.cf
Ho, Y.S. 2006. Review of second-order models for adsorption systems. Journal of Hazardous Materials. 136(3): 681-689. https://doi.org/10. 1016/j.jhazmat.2005.12.043
Ho, Y.S., and McKay, G. 1998. Sorption of dye from aqueous solution by peat. Chemical Engineering Journal. 70(2): 115-124. https://doi.org/10.1016/ S0923-0467(98)00076-1
Hussein, A., Larachi, F., Ziegler, D., and Alamdari, H. 2016. Effects of heat treatment and acid washing on properties and reactivity of charcoal. Biomass and Bioenergy. 90: 101-113. https://doi.org/10.1016/j.biombioe.
2016.03.041
Ken, B.S., and Nandi, B.K. 2018. Cleaning of high sulfur Indian coal by oil agglomeration and leaching. Energy Sources, Part A: Recovery, Utilization, and Environmental Effects. 40(9): 1027-1034. https://doi. org/10.1080/15567036.2017.1314395
Li, H., Huang, G., An, C., Hu, J., and Yang, S. 2013. Removal of tannin from aqueous solution by adsorption onto treated coal fly ash: kinetic, equilibrium, and thermodynamic studies. Industrial & Engineering ChemistryResearch. 52:15923–15931.https://doi.org/10.1021ie402054w
Liu, J., Jiang, X., Huang, X., and Wu, S. 2010. Morphological characterization of super fine pulverized coal particle, part 4, nitrogen adsorption and small angle x-ray scattering study. Energy Fuels. 24(5): 3072–3085. https://doi.org/10.1021/ef100142t
Liu, Z, Zhou, A., Wang, G., and Zhao, X. 2009. Adsorption behavior of methyl orange onto modified ultrafine coal powder. Chinese Journal of Chemical Engineering. 17(6): 942-948. https://doi.org/10.1016/S1004-9541(08)60300-6
López-Velandia, C., Moreno-Barbosa, J.J., Sierra-Ramirez, R., Giraldo, L., and Moreno-Piraján, J.C. 2014. Adsorption of volatile carboxylic acids on activated carbon synthesized from watermelon shells. Adsorption Science & Technology. 32(2-3): 227-242. https://doi.org/10.1260/0263-6174.32.2-3.227
Nadiye-Tabbiruka, M.S., and Lungani L. 2018. Removal of dyes from waste waters through adsorption using HCl activated coal. International Journal of Materials and Chemistry. 8(2): 23-30. https://doi.org/ 10.5923/j.ijmc.20180802.01
Nethaji, S., Sivasamy, A., and Mandal, A.B. 2013. Adsorption isotherms, kinetics and mechanism for the adsorption of cationic and anionic dyes onto carbonaceous particles prepared from Juglans regia shell biomass. International Journal of Environmental Science and Technology. 10(2): 231–242. https://doi.org/10.1007/s13762-012-0112-0
Pansuk, C., and Vinitnantharat, S. 2011. A comparative study of the adsorption of acid brown 75 and direct yellow 162 onto unmodified and surfactant modified granule developed from coal fly ash. 2011 2nd International Conference on Environmental Science and Technology. IPCBEE. 6. Singapore: Iacsit Press.
Sari, A., Mendil, D., Tuzen, M., and Soylak, M. 2008. Biosorption of Cd(II) and Cr(III) from aqueous solution by moss (Hylocomium splendens) biomass: equilibrium, kinetic and thermodynamic studies. The Chemical Engineering Journal. 144(1): 1-9. https://doi.org/ 10.1016/j.cej.2007.
12.020
Sharma, S.K., Mahiya, S., and Lofrano, G. 2017. Removal of divalent nickel from aqueous solutions using Carissa carandas and Syzygium aromaticum: isothermal studies and kinetic modelling. Applied Water Science. 7(4): 1855-1868. https://doi.org/10.1007/s13201-015-0359-y
Sun, D., Zhang, X., Wu, Y., Liu, X. 2010. Adsorption of anionic dyes from aqueous solution on fly ash. Journal of Hazardous Materials. 181(1-3): 335-342. https://doi.org/10.1016/j.jhazmat.2010.05.015
Sun, H., Wang, Y., Bian, Y., He, X., and Li, G. 2018. Adsorption behaviors of typical aromatic pollutants in biologically treated coking wastewater on powdered coal. Physicochemical Problems of Mineral Processing. 54(2): 496–504. https://doi.org/10.5277/ppmp1850
Sun, X., Xu, H., Wng, J., Ning, K., Huang, G., Yu, Y., and Ma, L. 2018. Kinetic research of quinoline, pyridine and phenol adsorption on modified coking coal. Physicochemical Problems of Mineral Processing. 54(3): 965-974. https://doi.org/10.5277/ppmp1898
Ting-chu, H. 2008. Adsorption of an acid dye onto coal fly ash. Fuel. 87(13-14): 3040-3045. https://doi.org/10.1016/j.fuel.2008.03.026
Ugurlu, M., and Karaoglu, M.H. 2011. Adsorption of ammonium from an aqueous solution by fly ash and aepiolite: isotherm, kinetic and thermodynamic analysis. Microporous and Mesoporous Materials. 139(1-3): 173-178. https://doi.org/10.1016/j.micromeso.2010.10.039
Wang, F., Lu, X., and Li, X. 2016. Selective removals of heavy metals (Pb(2+), Cu(2+), and Cd(2+)) from wastewater by gelation with alginate for effective metal recovery. Journal of Hazardous Materials. 308: 75-83. https://doi.org/10.1016/j.jhazmat.2016.01.021
Zhang, C., Li, J., Chen, Z., and Cheng, F. 2018. Factors controlling adsorption of recalcitrant organic contaminant from bio‐treated coking wastewater using lignite activated coke and coal tar‐derived activated carbon. Journal of Chemical Technology and Biotechnology. 93(1): 112-120. https://doi.org/10.1002/jctb.5328
Zhang, X.M., Zhang, Y., and Fan, D. 2014. Comparison of coal ash and activated carbon for removal of humic acid from aqueous solution Advanced Materials Research. 864-867: 1509-1512. https://doi.org/ 10.4028/www.scientific.net/AMR.864-867.1509
F. Akbar Jan1*, Naimat Ullah2, and Shahzad Hameed1
1Department of Chemistry, Bacha Khan University Chrasadda, Khyber- Pakhtunkhwa,24420 Pakistan
2Department of Chemistry, Qauid-i- Azam University Islamabad, 45320 Pakistan
*Corresponding Author. E-mail: fazal_akbarchem@yahoo.com
Total Article Views
Article history:
Received: October 23, 2018;
Revised: January 17, 2019;
Accepted: January 18, 2019

