
Artocarpus lakoocha Roxb. and Oxyresveratrol Inhibits Interleukin 6 Secretion Related with Insulin Resistance in 3T3-L1 Adipocytes
Saowalak Yomlar, Kanittaporn Trisat, and Nanteetip Limpeanchob*Published Date : December 12, 2023
DOI : https://doi.org/10.12982/NLSC.2024.005
Journal Issues : Number 1, January-March 2024
Abstract Chronic low-grade inflammation plays an important role in insulin resistance and the development of type 2 diabetes in obese people. Adipocytes, the most abundant cell type in adipose tissue, could produce various types of cytokines responsible for chronic inflammation. The purpose of the present study was to investigate the preventive effect of oxyresveratrol and Puag-Haad, which is the oxyresveratrol-enriched aqueous extract from Artocarpus lakoocha Roxb., on the inflammation and insulin resistance demonstrated by adipocytes. The release of pro-inflammatory cytokines, including TNF-α, IL-1β, and IL-6, were monitored to evaluate the inflammatory status of the adipocyte cells during the 3T3-L1 adipocytes maturation period. Intracellular lipid accumulation and glucose uptake were measured to assess adipogenicity and insulin sensitivity status of mature adipocytes, respectively. The results showed that 3T3-L1 adipocytes substantially secreted IL-6 but not TNF-α, IL-1β. Further, IL-6 secretion was correlated with lipid quantity accumulated in the adipocytes. Oxyresveratrol and Puag-Haad predominantly exhibited anti-inflammatory activity in a dose-dependent manner, inhibiting IL-6 release but showed less effect on lipid accumulation. 3T3-L1 adipocytes treated with Puag-Haad (40 µg/mL) for 15 days showed significant decreases in fat accumulation and IL-6 secretion and promoted the glucose uptake. These outcomes indicate an improvement in insulin sensitivity. Taken all together, the data indicates that Puag-Haad is an interesting natural substance that should be further studied for its preventive effect on obese-mediated inflammation and the consequences of insulin resistance and type 2 diabetes.
Keywords: Artocarpus lakoocha Roxb., Oxyresveratrol, Pro-inflammatory cytokine, Insulin resistance, Adipocyte
Funding: The authors are grateful for research co-funding provided by the Center of Excellence for Innovation in Chemistry (PERCH-CIC).
Citation: Yomlar, S., Trisat, K., and Limpeanchob, N. 2024. Artocarpus lakoocha Roxb. and oxyresveratrol inhibits interleukin 6 secretion related with insulin resistance in 3T3-L1 adipocytes. Natural and Life Sciences Communications. 23(1): e2024005.
INTRODUCTION
Diabetes Mellitus (DM) is one of the largest global health emergencies of the 21st century. The International Diabetes Federation estimates the number of patients with diabetes will reach 642 million by 2040, a 55% increase from 2015 (Ogurtsova et al., 2017). Globally, between 90–95% of cases are type 2. Type2 (T2DM) is characterized by insulin resistance which is a pathological condition whereby cells fail to respond normally to insulin which leads to high blood glucose levels (Schinner et al., 2005). Chronic inflammation is an important link between obesity and insulin resistance. During the development of obesity, macrophage infiltration in adipose tissue contributes to inducing the state of low-grade inflammation, and consequently the reduction in insulin sensitivity of insulin responsive cells such as adipocytes and muscle cells (Van Greevenbroek et al., 2013). In obese subjects, pro-inflammatory cytokines, such as tumor necrosis factor alpha (TNF-α), interleukin 1 beta (IL-1β), and interleukin 6 (IL-6), were higher than in lean subjects (Nehete et al., 2014; Popko et al., 2010; Vugic et al., 2020). Cytokine levels were also found to be involved with the phosphorylation of insulin receptor substrate-1 (IRS-1) and glucose uptake indicating an insulin resistance condition (Fantuzzi, 2005; Meng et al., 2010). Thus, modulation of inflammatory responses in adipose tissue may consequently improve insulin sensitivity, making inflammatory responses a potential target to attenuate insulin resistance and T2DM development.
In Thailand, Artocarpus lakoocha Roxb. is locally known as “Ma-Haad”. A product prepared by extracting the heartwood of this plant with boiling water, known as “Puag-Haad” has been traditionally used as an anti-helminthic (Preyavichyapugdee et al., 2016). The major compound found in Puag-Haad is oxyresveratrol which is highly enriched in the range of 780.1 and 837.5 mg/g (Maneechai et al., 2009). Anti-inflammatory activities of oxyresveratrol-enriched A. lakoocha extract (Hankittichai et al., 2020) and oxyresveratrol (Lee et al., 2015; Wei et al., 2017) were demonstrated as suppressing lipopolysaccharide (LPS)-induced production of cytokines and mediators in RAW264.7 murine macrophage cell lines. Oxyresveratrol was also reported as a potential agent for modulating metabolic conditions such as obesity and diabetes. Oxyresveratrol increased insulin secretion in INS-1 pancreatic β-cell line and ameliorated insulin resistance in mice fed with high-fat diet (Park et al., 2017). In streptozotocin-induced diabetic mice, oxyresveratrol reduced fasting plasma glucose and increased glucose transporter 2 (GLUT2) levels in hepatic cells (Ahn et al., 2017). Oxyresveratrol supplementation improves various biochemical parameters as well as the expression of proteins related to lipid and glucose metabolism in high-fat diet-fed mice (Choi et al., 2018; Tan et al., 2017). Oxyresveratrol was reported to suppress lipid accumulation and induced thermogenic gene expression in adipocyte cell lines (Choi et al., 2018). According to these reports, oxyresveratrol exhibited anti-inflammation properties and other beneficial effects in lipid and glucose metabolism dysfunctions. The effect of oxyresveratrol and oxyresveratrol-enriched extract (Puag-Haad) on pro-inflammation and insulin resistance status in cultured adipocyte cells was therefore the focus of the present study in which the effect of oxyresveratrol and Puag-Haad on the secretion of pro-inflammatory cytokines including TNF-α, IL-1β, and IL-6 in differentiated 3T3-L1 adipocytes were investigated. Also, their effect on insulin resistance was assessed by measuring the insulin-induced glucose uptake in fully mature adipocytes.
MATERIALS AND METHODS
Chemical
2-NBDG (2-(N-(7-Nitrobenz-2-oxa-1,3-diazol-4-yl)amino)-2Deoxyglucose) was purchased from Invitrogen™ (Thermo Fisher, Waltham, MA). 3-Isobutyl-1-methylxanthine (IBMX), dexamethasone, Oli Red O, isopropanol, 3-[4,5-dimethylthiazol-2-yl]-2,3-diphenyl tetrazolium bromide (MTT), insulin, and oxyresveratrol were purchased from Sigma-Aldrich (St. Louis, MO). Dulbecco modified Eagle medium (DMEM), calf bovine serum (CBS), fetal bovine serum (FBS), trypsin/EDTA, and penicillin/streptomycin were purchased from GIBCO (Carlsbad, CA). Mouse IL-1 beta/IL-1F2 DuoSet ELISA kit, Mouse IL-6 DuoSet ELISA kit, and Mouse TNF-alpha DuoSet ELISA kit were purchased from R&D systems (Minneapolis, MN). Puag-Haad was obtained from Origin Plant Co., Ltd., (Samutprakan, Thailand), which had been prepared by boiling A. lakoocha wood chips in water, collecting the bubble foam, and finally drying the collected material. Puag-Haad used in this study contained 5.67% phenolic compounds and traces of flavonoids while the main content was oxyresveratrol (~65%) by HPLC analysis (Wongon and Limpeanchob, 2020).
3T3-L1 cell culture and differentiation
Mouse 3T3-L1 pre-adipocytes (CL-173™) were purchased from American Type Culture Collection (ATCC, Manassas, VA). 3T3-L1 cell culture preparation and differentiation were performed with slight modifications (Trisat et al., 2020). 3T3-L1 pre-adipocytes were grown in high glucose-DMEM supplemented with 10% CBS and 100 U/mL penicillin and 100 μg/mL streptomycin. Cells were incubated at 37°C in a humidified incubator with 5% CO2. To induce cell differentiation, 3T3-L1 pre-adipocytes were cultured in differentiation medium (high glucose-DMEM supplemented with 10% FBS, 1 µM dexamethasone, 0.5 mM IBMX, and 10 µg/mL insulin) for 3 days. After day 3, the cells were cultured with maintenance medium (high glucose-DMEM supplemented with 10% FBS and 10 µg/mL insulin) for a further 15 days in which the cultured medium was replaced every 3 days.
Cell viability determination
3T3-L1 cell viability was analyzed using MTT assay (Duangjai et al., 2018). Briefly, 3T3-L1 cells were seeded at 3×103 cells per well in 96-well plates. After differentiation, the cells were treated with various concentrations of oxyresveratrol (1-100 µM) or Puag-Haad (2-40 µg/mL) for either 24 h or 15 days. Two hours prior to the end of the treatment, MTT was added into the medium at 0.5 mg/mL final concentration. The formazan crystals produced in viable cells were dissolved in 200 µL DMSO, and the absorbance was measured at 595 nm wavelength using a microplate reader.
Determination of lipid accumulation in adipocyte
Lipid accumulation in the cultured 3T3-L1 cells was determined by Oli Red O staining in both the absence and presence of oxyresveratrol or Puag-Haad. Oli Red O staining was performed according to previous reports (Che et al., 2021; Trisat et al., 2020). After treatment, the cells were washed with PBS, fixed with 10% v/v formalin for at least 1 h, rinsed with 60% isopropanol, and stained with 3.5 mg/mL Oil Red O solution for 10 min. After three times washing with PBS, the stained oil droplets inside the cells were lysed with 100% isopropanol and the absorbance was read by a microplate reader at wavelength 510 nm.
Determination of pro-inflammatory cytokines by ELISA
3T3-L1 cells were treated with various concentrations of oxyresveratrol or Puag-Haad for indicative time periods depending on the experimental conditions. The medium was collected, centrifuged, and the supernatant was tested for pro-inflammatory cytokines (TNF-α, IL-1β and IL-6) using ELISA kits. The ELISA procedures were conducted according to the instruction manual provided in the assay kit purchased.
Determination of glucose uptake in adipocytes
3T3-L1 cells were cultured onto 96-well clear bottom black plates and treated with various concentrations of oxyresveratrol or Puag-Haad for indicative time periods depending on the experimental conditions. Determination of glucose uptake in adipocytes was performed using a 2-NBDG fluorescent assay, adapted from a previous study (Choi et al., 2021). Prior to measuring the glucose uptake, the culture medium was replaced with glucose free DMEM containing 1 µg/mL insulin and 25 µM 2-NBDG, a fluorescent glucose analog. After incubating for 3 h, the medium was removed, the cells were washed with PBS three times, and the fluorescence was measured by a microplate reader (485 nm excitation and 535 nm emission wavelengths).
Statistical analysis
All data are represented as mean ± standard error of the mean (SEM) of at least three separate experiments. The data were analyzed by analysis of variance (ANOVA) followed by the least significant different (LSD) tests and paired-sample t-Test. P ˂ 0.05 was considered statistically significant.
RESULTS
Lipid accumulation and pro-inflammatory cytokines release during 3T3-L1 adipocyte maturation
Throughout the 15 days of 3T3-L1 adipocyte maturation (Figure 1A), the accumulation of intracellular lipids was determined by Oil red O staining (Figure 1B). The level of intracellular lipids continuously increased, and the day 3 value was more than doubled by day 9 (Figure 1C). The secretion of pro-inflammatory cytokines; TNF-α, IL-1β, and IL-6 was measured every 3 days over the 15-day period (Figure 2A). Without lipopolysaccharide (LPS) induction, the mature adipocytes secreted IL-6 in significant levels after day 9 of maturation (Figure 2D). In contrast to IL-6, the secretions of TNF-α and IL-1β did not obviously appear during the 15 days period of adipocyte maturation (Figures 2B and 2C). As well, the secretion of IL-6 from the mature adipocytes seems to correlate with the degree of intracellular lipid accumulation (Figure 3). These data suggest that mature adipocytes are filled with lipids and express certain properties of pro-inflammatory cells.
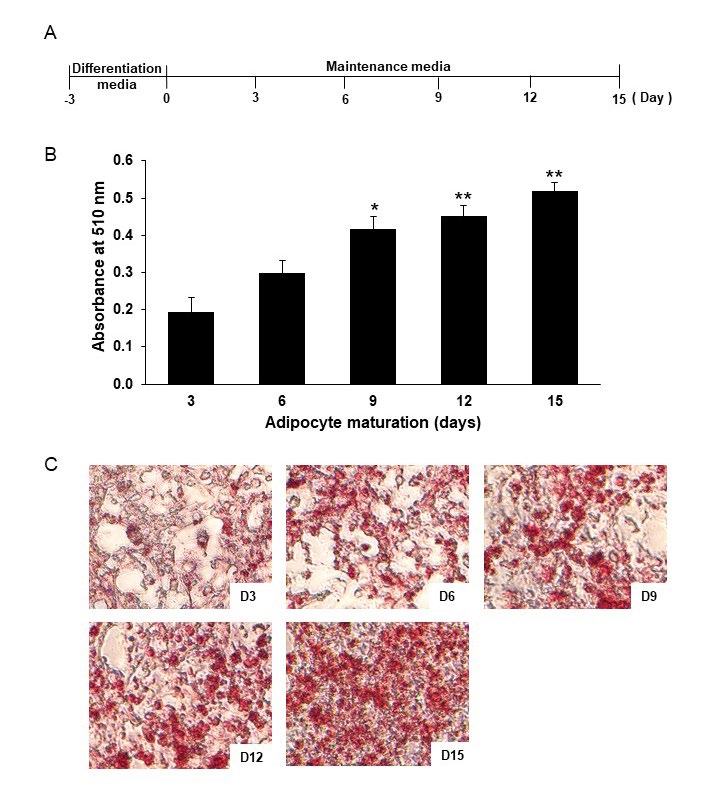
Figure 1. Lipid accumulation of 3T3-L1 adipocytes. Schematic outline of the experimental setup: after differentiation, 3T3-L1 adipocytes were cultured in maintenance media which were replaced every 3 days (A). Lipid accumulations were determined by Oil Red O staining and observed under a microscope (40x) (C) and quantitatively analyzed by dissolving lipid droplets with isopropanol and measuring the absorbance at 510 nm (B). Data are expressed as mean ± SEM (n = 4-6). *P ˂ 0.05, **P ˂ 0.01 compared with day 3.
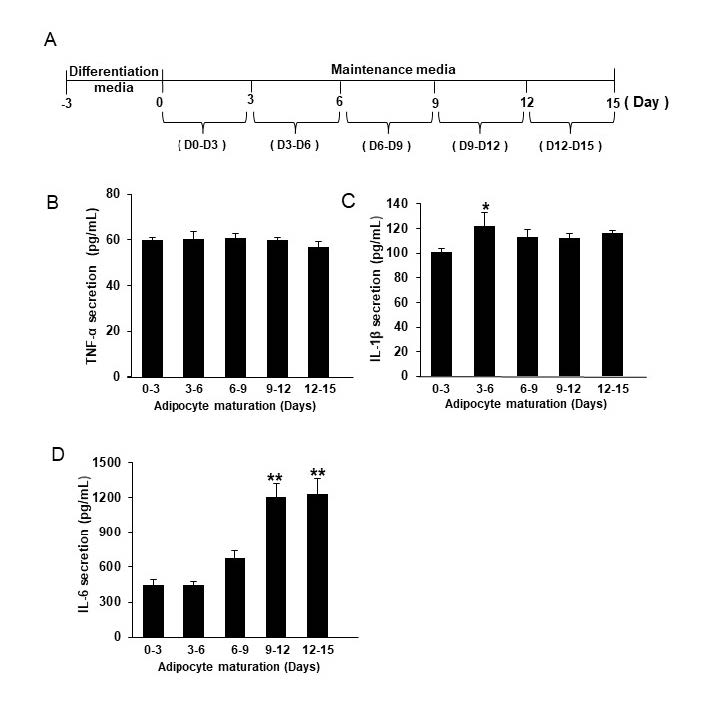
Figure 2. The release of pro-inflammatory cytokines from 3T3-L1 adipocytes. Schematic outline of the experimental setup: after differentiation, 3T3-L1 adipocytes were cultured in maintenance media which were replaced every 3 days (A). TNF-alpha (B), IL-1β beta (C), and IL-6 (D) were measured from those removed media. Data are expressed as mean ± SEM (n = 4-6). *P ˂ 0.05, **P ˂ 0.01 compared with D3-6.
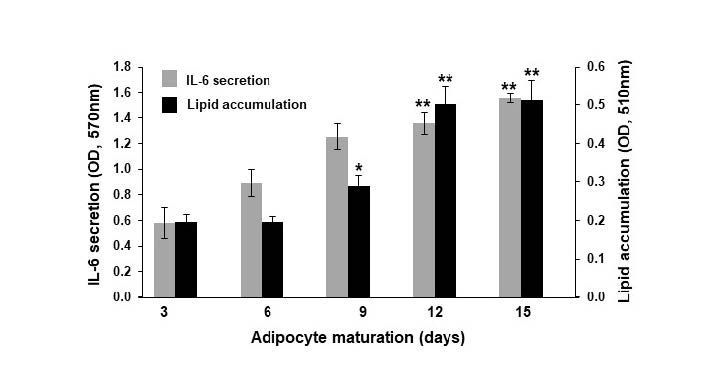
Figure 3. Lipid accumulation and IL-6 secretion of 3T3-L1 adipocytes during 15 days of maturation. Data are expressed as mean ± SEM (n = 4-6). *P ˂ 0.05, **P ˂ 0.01 compared with data at day 3.
Oxyresveratrol and Puag-Haad suppressed IL-6 secretion from mature 3T3-L1 adipocytes
The releases of IL-6, measured at days 12-15 (Figure 4B), were suppressed in a dose-dependent manner in the presence of oxyresveratrol or Puag-Haad for the entire 15 days of 3T3-L1 adipocyte maturation (Figure 4A). In the tests, all concentrations of oxyresveratrol significantly inhibited lipid accumulation in the adipocytes whereas Puag-Haad reduced the accumulated lipids level at 40 µg/mL (Figure 4C). During the 15 days of the incubation period, cell viabilities were higher than 80% in the presence of either tested compound, indicating a nontoxic effect on the adipocytes (Figure 4D). These data suggest that oxyresveratrol and Puag-Haad both demonstrate a significant anti-pro-inflammatory activity whereas their anti-adipogenesis or lipid accumulation reduction activity was less intense.
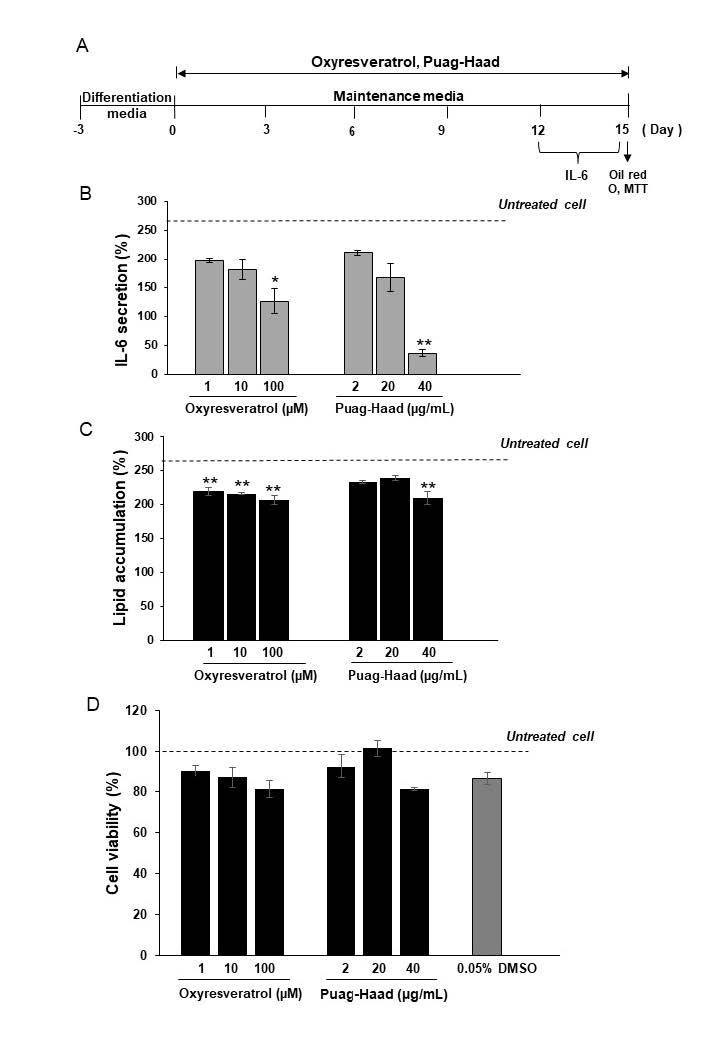
Figure 4. The effect of oxyresveratrol and Puag-Haad on lipid accumulation and IL-6 release in mature 3T3-L1 adipocytes. Schematic outline of the experimental setup: after differentiation, 3T3-L1 adipocytes were cultured in maintenance media in the presence of oxyresveratrol or Puag-Haad in which media were replaced every 3 days (A). IL-6 were measured from D12-15 media by ELISA kit (B). Lipid accumulations were determined by Oil Red O staining (C) and cell viability were assessed by MTT assay (D). Data are expressed as mean ± SEM (n = 4-6). *P ˂ 0.05, **P ˂ 0.01 compared with untreated cells.
Effect of oxyresveratrol and Puag-Haad on glucose uptake into mature 3T3-L1 adipocytes
During 3T3-L1 adipocyte maturation, glucose was increasingly taken-up into the adipocytes over the 15 days period, with varying levels of up-take on different days (Table 1), with the highest increase (40%) occurring on day 6 of maturation. The insulin-stimulated glucose uptake was reduced at and after 9 days of maturation (Table 1), implying that fully mature adipocytes lose their insulin sensitivity over time.
Table 1. Glucose uptake of adipocytes at different stage of cell maturation.
|
Age of maturation (days) |
Fluorescent intensity |
Effect of insulin (% increase) |
|
|
No insulin |
Insulin |
||
|
3 |
645 ± 8 |
711 ± 35 |
11 ± 11 |
|
6 |
961 ± 66 |
1,345 ± 92** |
41 ± 3 |
|
9 |
989 ± 26 |
1,176 ± 45** |
19 ± 2 |
|
12 |
1,173 ± 19 |
1,423 ± 23** |
21 ± 2 |
|
15 |
1,470 ± 65 |
1,688 ± 67** |
16 ± 3 |
Note: Glucose uptake was determined using fluorescent glucose derivative. n = 3-15, *P ˂ 0.05, **P ˂ 0.01; no insulin vs insulin
After 3T3-L1 adipocytes were pre-treated with oxyresveratrol or Puag-Haad for 15 days maturation period, both of the pre-treated samples showed a higher uptake of glucose than the untreated sample. Significant increases were observed in the oxyresveratrol pre-treated sample, at 25 and 50 µM concentrations of oxyresveratrol (Table 2). The Puag-Haad pre-treated sample showed only a small increase in glucose uptake, but this was not significant. In insulin-stimulated condition, Puag-Haad showed increased insulin sensitivity at 40 µg/mL concentration only, which was a significant increase with a 28% glucose uptake (Table 2). The oxyresveratrol pre-treated sample showed no increase whatever in the presence of insulin. It should be noted that the uptake of glucose and insulin sensitivity were monitored over a 3 h period with the uptake of fluorescent glucose derivatives being measured in the absence of both oxyresveratrol and Puag-Haad. So that the effects on glucose uptake were expected to be the result of changes on cellular response occurred during 15 days of oxyresveratrol or Puag-Haad pre-treatment.
To investigate the direct effect of oxyresveratrol and Puag-Haad on both the uptake of glucose and insulin sensitivity, mature adipocytes: cultured for 15 days and which had not been pre-treated with oxyresveratrol and Puag-Haad, were monitored for 3 h with oxyresveratrol and Puag-Haad added. The results showed that Puag-Haad at a concentration of 40 µg/mL significantly promoted glucose uptake. However, there was no significant insulin sensitivity observed (Table 3).
Table 2. Glucose uptake of adipocytes pre-treated with oxyresveratrol or Puag-Haad for 15 days.
|
Conditions |
Concentrations |
Fluorescent intensity |
Effect of insulin (%increase) |
|
|
No insulin |
Insulin |
|||
|
Untreated cells |
1,470 ± 65 |
1,688 ± 67** |
16 ± 3 |
|
|
Oxyresveratrol (µM) |
25 |
1,844 ± 178# |
1,922 ± 197 |
4 ± 2 |
|
50 |
1,891 ± 119## |
1,921 ± 73 |
3 ± 5 |
|
|
100 |
1,468 ± 88 |
1,559 ± 87 |
8 ± 8 |
|
|
Puag-Haad (µg/mL) |
10 |
1,751 ± 229 |
1,869 ± 81 |
13 ± 14 |
|
20 |
1,772 ± 111 |
2,028 ± 158# |
15 ± 8 |
|
|
40 |
1,549 ± 86 |
1,986 ± 157** |
28 ± 3 |
|
Note: Glucose uptake was determined at the end of 15 days pre-treatment with oxyresveratrol or Puag-Haad. n = 5-14, *P ˂ 0.05, **P ˂ 0.01; no insulin vs insulin, #P ˂ 0.05, ##P ˂ 0.01; untreated vs treated cells
Table 3. Direct effect of oxyresveratrol and Puag-Haad on the glucose uptake of adipocytes.
|
Conditions |
Concentrations |
Fluorescent intensity |
Effect of insulin (%increase) |
|
|
No insulin |
Insulin |
|||
|
Untreated cells |
1,470 ± 65 |
1,688 ± 67** |
16 ± 3 |
|
|
Oxyresveratrol (µM) |
25 |
1,639 ± 124 |
1,680 ± 117 |
3 ± 6 |
|
50 |
2,011 ± 192## |
1,820 ± 142 |
-8 ± 8 |
|
|
100 |
1,531 ± 91 |
1,517 ± 73 |
-1 ± 3 |
|
|
Puag-Haad (µg/mL) |
10 |
1,400 ± 83 |
1,504 ± 27 |
8 ± 4 |
|
20 |
1,519 ± 165 |
1,593 ± 134 |
6 ± 5 |
|
|
40 |
1,863 ± 186# |
1,996 ± 191# |
9 ± 8 |
|
Note: Glucose uptake was determined at the end of 15 days cell maturation, oxyresveratrol or Puag-Haad was present during 3 h of glucose uptake assay. n = 5-14, *P ˂ 0.05, **P ˂ 0.01; no insulin vs insulin, #P ˂ 0.05, ##P ˂ 0.01; untreated vs treated cells
DISCUSSION
Obesity is associated with a state of chronic low-grade inflammation which is an important mechanism leading to insulin resistance (Trayhurn and Wood, 2004; Van Greevenbroek et al., 2013). Previous studies have reported that high fat diet-induced obese mice developed insulin resistance and exhibited increased expression of several cytokines such as IL-6 and TNF-alpha (Meng et al., 2010). Serum concentration of both IL-6 and TNF-alpha in obese human subjects were found to be high (Popko et al., 2010). In addition to energy storage, adipose tissue plays a role in immunity functions because it contains various immune cells (Makki et al., 2013). As well as immune cells, adipocytes also could secrete various types of pro-inflammatory cytokines (Fantuzzi, 2005; Makki et al., 2013). In the present study, fully mature 3T3-L1 adipocytes, without endotoxins-induction, dramatically secreted IL-6, and the levels of released IL-6 were positively correlated with the degree of intracellular lipid accumulation. In agreement with a previous study, (Ahmed and Si, 2021) differentiated 3T3-L1 adipocytes increasingly secreted IL-6 over increasing length of culturing periods. In the present study, the secretion of TNF-α and IL-1β from adipocytes was not clearly observed which suggests that IL-6 is the main cytokine released from fatty adipocytes. A previous study reported that adipocytes, especially from omental fat, released substantial quantities of IL-6 (Fried et al., 1998). In human subjects, circulating levels of IL-6 are expressed predominantly in adipocytes, correlated with adiposity, which is associated with weight loss (Makki et al., 2013). Greater amounts of IL-6 than TNF-α and IL-1β were released in obese subjects with a BMI >40 (Kern et al., 2001; Fain et al., 2004) and when IL-6 receptors were highly expressed in the subcutaneous adipose tissues of obese subjects (Sindhu et al., 2015). These observations indicate that IL-6 could be the major pro-inflammatory cytokine indicators of the inflammatory status of fatty adipocytes and are associated with the development of insulin resistance.
In the present study, IL-6, released from fully mature adipocytes, were demonstrated as being important components responsible for the reduction of insulin sensitivity of these cells. The influence of IL-6 on insulin resistance has been demonstrated in both primary hepatocytes and HepG2 cell lines, (Senn et al., 2002) as well as in mice models (Klover et al., 2003). IL-6-treated adipocytes diminished protein expression of IR-β subunit, IRS-1, and glucose transporter-4 (GLUT4) supporting its roles in insulin resistance induction in adipose cells (Lagathu et al., 2003).
A. lakoocha extract was previously reported to inhibit LPS-mediated production and secretion of cytokines and chemokines from macrophage cell (Hankittichai et al., 2020). A. lakoocha is known for its high content of stilbenoids, especially oxyresveratrol, which are predominant in the heartwood of the plant. Oxyresveratrol exhibits anti-inflammatory effect by inhibiting cytokine production in LPS-stimulated macrophages (Lee et al., 2015) and also showed anti-obese effects in high-fat diet fed obese mice and suppressed lipid accumulation in the 3T3-L1 adipocytes(Choi et al., 2018). However, the direct anti-inflammatory effect of A. lakoocha and oxyresveratrol in adipocytes has not been clearly demonstrated. The present study showed that the release of pro-inflammatory cytokine IL-6 from mature 3T3-L1 adipocytes is dose-dependently suppressed by oxyresveratrol and Puag-Haad. In LPS-stimulated RAW264.7 cells, oxyresveratrol reduced mRNA and protein expressions of IL-6 by suppressing the phosphorylation of IκBα and translocation p65 NFκB subunit into the nucleus (Lee et al., 2015; Wei et al., 2017). Oxyresveratrol also suppressed the phosphorylation of protein kinase B (Akt) and c-Jun N-terminal kinases (JNK) and p38 mitogen-activated protein kinases (MAPKs) in LPS-induced inflammatory responses (Lee et al., 2015). A. lakoocha ethanolic extract suppressed LPS-stimulated macrophage through the inhibition of NFκB and MAPK signaling pathways (Hankittichai et al., 2020). The evidence on immune cells demonstrates that the inhibitory activity of oxyresveratrol and Puag-Haad on IL-6 in mature adipocytes could be also responsible for the inhibition of MAPK and/or NF-κB signaling pathways.
In the present study, by the development of insulin resistance in fully mature adipocytes, cultured for 15 days was the cause of reduced insulin-induced glucose uptake. Pro-inflammatory cytokine IL-6 was also thought to play a critical role in decreasing insulin response of adipocytes. Glucose uptake and glucose consumption of insulin-responsive tissues; hepatocyte, myocyte, and adipocyte, were decreased after TNF-α and IL-6 treatments (Lagathu et al., 2003; Yang et al., 2015). In adipose tissue biopsies, collected from insulin-resistant individuals, glucose transporter-4 (GLUT4) was down regulated, and the secretion of IL-6 was increased compared to insulin-sensitive adipose tissues (Almuraikhy et al., 2016). The main role of GLUT4 is to provide insulin-stimulated glucose uptake into insulin-responsive tissues. The disruption of the GLUT4 resulted in the reduction of 2-deoxyglucose uptake into skeletal muscle by 85–90% and into white adipose tissue by 65% in mice (Kotani et al., 2004).
The increase in insulin sensitivity of mature adipocytes was also demonstrated in the present study, after the mature adipocytes had been treated with Puag-Haad. The direct effect of Puag-Haad on glucose uptake in mature adipocytes was also observed. Puag-Haad at the concentration of 40 µg/mL, exhibited the highest potential in terms of suppression of IL-6 release and lipid accumulation, as well as promoting insulin sensitivity. This concentration of Puag-Haad is equivalent to 106 µM oxyresveratrol, but oxyresveratrol at 100 µM did not clearly affect insulin sensitivity and glucose uptake in a adipocyte model. Thus, in addition to oxyresveratrol, other bioactive components in Puag-Haad such as artocarpin, norartocarpin, norcycloartocarpin, cycloartocarpin, and resorcinol might also play some role in such activities (Gautam, 2014). In high fat-diet mice, oxyresveratrol upregulated mRNA GLUT4 levels in both visceral fat and muscle cell and consequently improved blood glucose levels (Tan et al., 2017). So that oxyresveratrol and oxyresveratrol-enriched extracts have been shown to have high potential and should be required in further studies.
CONCLUSION
Fully mature 3T3-L1 adipocytes substantially secreted IL-6 but not TNF-α and IL-1β. The secretion of IL-6 is well correlated with the degree of intracellular lipid accumulation in the adipocytes which suggests the inflammatory status of fatty adipocyte cells. Oxyresveratrol and Puag-Haad exhibited anti-inflammatory activity in adipocytes through the inhibition of IL-6 release. Puag-Haad showed its potential to enhance insulin sensitivity since it promoted the insulin-dependent glucose uptake in fully mature 3T3-L1 adipocytes. Oxyresveratrol and other ingredients in Puag-Haad might be responsible for such activities and their potential in the prevention of inflammation and the subsequent insulin resistance in obese subjects should be further investigated.
ACKNOWLEDGEMENTS
The authors would like to thank the Center of Excellence for Innovation in Chemistry (PERCH-CIC) and Naresuan University for financial support to the graduate student.
AUTHOR CONTRIBUTIONS
Saowalak Yomlar conducted the experiments, performed the statistical analysis, and wrote first draft of the manuscript. Kanittaporn Trisat assisted in conducting certain experiments and revised the manuscript. Nanteetip Limpeanchob supervised in conducting the experiments and edited the manuscript. All authors have read and approved of the final manuscript.
CONFLICT OF INTEREST
The authors declare that they hold no competing interests.
REFERENCES
Ahmed, B. and Si, H. 2021. The aging of adipocytes increases expression of pro-inflammatory cytokines chronologically. Metabolites. 11(5): 292.
Ahn, E., Lee, J., Jeon, Y.H., Choi, S.W. and Kim, E. 2017. Anti-diabetic effects of mulberry (Morus alba L.) branches and oxyresveratrol in streptozotocin-induced diabetic mice. Food Science and Biotechnology. 26(6): 1693-1702.
Almuraikhy, S., Kafienah, W., Bashah, M., Diboun, I., Jaganjac, M., Al-Khelaifi, F., Abdesselem, H., Mazloum, N.A., Alsayrafi, M., Mohamed-Ali, V. et al. 2016. Interleukin-6 induces impairment in human subcutaneous adipogenesis in obesity-associated insulin resistance. Diabetologia. 59(11): 2406–2416.
Che, L., Ren, B., Jia, Y., Dong, Y., Wang, Y., Shan, J., and Wang, Y. 2021. Feprazone displays antiadipogenesis and antiobesity capacities in in vitro 3 t3-l1 cells and in vivo mice. ACS Omega. 6(10): 6674-6680.
Choi, E., Baek, S., Baek, K. and Kim, H.K. 2021. Psidium guajava L. leaf extract inhibits adipocyte differentiation and improves insulin sensitivity in 3T3-L1 cells. Nutrition Research Practice. 15(5): 568-578.
Choi, J.H., Song,N-J., Lee, A.R., Lee,D.H., Seo,M-J., Kim, S., Chang, S-H., Yang, D.K., Hwang, Y-J., Hwang, K-A., et al. 2018. Oxyresveratrol increases energy expenditure through foxo3a-mediated ucp1 induction in high-fat-diet-induced obese mice. International Journal of Inflammation. 20(1): 26.
Duangjai, A., Nuengchamnong, N., Suphrom, N., Trisat, K., Limpeanchob, N., and Saokaew, S. 2018. Potential of coffee fruit extract and quinic acid on adipogenesis and lipolysis in 3T3-L1 adipocytes. Kobe Journal of Medical Sciences. 64(3): E84-E92.
Fain, J.N., Madan, A.K., Hiler, M.L., Cheema, P. and Bahouth, S.W. 2004. Comparison of the release of adipokines by adipose tissue, adipose tissue matrix, and adipocytes from visceral and subcutaneous abdominal adipose tissues of obese humans. Endocrinology. 145(5): 2273-2282.
Fantuzzi, G. 2005. Adipose tissue, adipokines, and inflammation. Journal of Allergy and Clinical Immunology. 115(5): 911-919.
Fried, S.K., Bunkin, D.A., and Greenberg, A.S. 1998. Omental and subcutaneous adipose tissues of obese subjects release interleukin-6: depot difference and regulation by glucocorticoid. Journal of Clinical Endocrinology and Metabolism. 83(3): 847-850.
Gautam, P. 2014. Artocarpus lakoocha Roxb. : An overview. Complementary and Alternative Medicine. 1: 10-14.
Hankittichai, P., Buacheen, P., Pitchakarn, P., Takuathung, M.N., Wikan, N., Smith, D.R., Potikanond, S., and Nimlamool, W. 2020. Artocarpus lakoocha extract inhibits LPS-induced inflammatory response in RAW 264.7 macrophage Cells. International Journal of Inflammation. 21(4): 1355.
Kern, P.A., Ranganathan, S., Li, C., Wood, L., and Ranganathan, G. 2001. Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. American Journal of Physiology-Endocrinology and Metabolism. 280(5): E745-E751.
Klover, P.J., Zimmers, T.A., Koniaris, L.G., and Mooney, R.A. 2003. Chronic exposure to interleukin-6 causes hepatic insulin resistance in mice. Diabetes. 52(11): 2784-2789.
Kotani, K., Peroni, O.D., Minokoshi, Y., Boss, O., and Kahn, B.B. 2004. GLUT4 glucose transporter deficiency increases hepatic lipid production and peripheral lipid utilization. Journal of Clinical Investigation. 114(11): 1666-1675.
Lagathu, C., Bastard, J-P., Auclair, M., Maachi, M., Capeau, J., and Caron, M. 2003. Chronic interleukin-6 (IL-6) treatment increased IL-6 secretion and induced insulin resistance in adipocyte: Prevention by rosiglitazone. Biochemical and Biophysical Research Communications. 311(2): 372-379.
Lee, H.S., Kim, D.H., Hong, J.E., Lee, J.Y., and Kim, E.J. 2015. Oxyresveratrol suppresses lipopolysaccharide-induced inflammatory responses in murine macrophages. Human and Experimental Toxicology. 34(8): 808-818.
Makki, K., Froguel, P., and Wolowczuk, I. 2013. Adipose tissue in obesity-related inflammation and insulin resistance: Cells, cytokines, and chemokines. ISRN Inflammation. 2013: 139239.
Maneechai, S., Likhitwitayawuid, K., Sritularak, B., Palanuvej, C., Ruangrungsi, N., and Sirisa-Ard, P. 2009. Quantitative analysis of oxyresveratrol content in Artocarpus lakoocha and 'Puag-Haad'. Medical Principles and Practice. 18(3): 223-227.
Meng, R., Zhu, D.L., Bi, Y., Yang, D.H., and Wang, Y.P. 2010. Apocynin improves insulin resistance through suppressing inflammation in high-fat diet-induced obese mice. Mediators of Inflammation. 2010: 858735.
Nehete, P., Magden, E.R., Nehete, B., Hanley, P.W., and Abee, C.R. 2014. Obesity related alterations in plasma cytokines and metabolic hormones in chimpanzees. International Journal of Inflammation. 2014: 856749.
Ogurtsova, K., da Rocha Fernandes, J.D., Huang, Y., Linnenkamp, U., Guariguata, L., Cho, N.H., Cavan,D., Shaw, J.E., and Makaroff. L.E. 2017. IDF diabetes atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Research and Clinical Practice. 128: 40-50.
Park, S.Y., Jin, B., Shin, J.H., Adisakwattana, S., and Kwon, O. 2017. Standardized Mori ramulus extract improves insulin secretion and insulin sensitivity in C57BLKS/J db/db mice and INS-1 cells. Biomedicine & Pharmacotherapy. 92: 308-315.
Popko, K., Gorska, E., Stelmaszczyk-Emmel, A., Plywaczewski, R., Stoklosa, A., Gorecka, D., Pyrzak, B., and Demkow, U. 2010. Proinflammatory cytokines Il-6 and TNF-α and the development of inflammation in obese subjects. European Journal of Medical Research. 15 (2): 120-122.
Preyavichyapugdee, N., Sangfuang, M., Saowapark Chaiyapum, S., Sriburin, S., Pootaeng-on, Y., Chusongsang, P., Jiraungkoorskul, W., Preyavichyapugdee, M., and Sobhon, P. 2016. Schistosomicidal activity of the crude extract of Artocarpus lakoocha. Southeast Asian Journal of Tropical Medicine and Public Health. 47(1): 1-15.
Schinner, S., Scherbaum, W.A., Bornstein, S.R. and Barthel, A. 2005. Molecular mechanisms of insulin resistance. Diabetic medicine: A journal of the British Diabetic Association. 22(6): 68-75.
Senn, J.J., Klover, P.J., Nowak, I.A. and Mooney, R.A. 2002. Interleukin-6 induces cellular insulin resistance in hepatocytes. Diabetes. 51(12): 3391-3399.
Sindhu, S., Thomas, R., Shihab, P., Sriraman, D., Behbehani, K., and Ahmad, R. 2015. Obesity is a positive modulator of IL-6R and IL-6 expression in the subcutaneous adipose tissue: Significance for metabolic inflammation. PLoS One. 10(7): e0133494.
Tan, H.Y., Tse, I.M., Li, E.T., and Wang, M. 2017. Oxyresveratrol supplementation to C57bl/6 mice fed with a high-fat diet ameliorates obesity-associated symptoms. Nutrients. 9(2): 147.
Trayhurn, P. and Wood, I.S. 2004. Adipokines: Inflammation and the pleiotropic role of white adipose tissue. British Journal of Nutrition. 92(3): 347-355.
Trisat, K., Yomlar, S. and Limpeanchob, N. 2020. Role of GABA and its receptors in anti-adipogenesis in cultured adipocytes. Songklanakarin Journal of Science & Technology. 42(5): 1053-1058.
Van Greevenbroek, M.M., Schalkwijk, C.G. and Stehouwer, C.D. 2013. Obesity-associated low-grade inflammation in type 2 diabetes mellitus: causes and consequences. Netherlands Journal of Medicine. 71(4): 174-187.
Vugic, L., Colson, N., Nikbakht, E., Gaiz, A., Holland, O.J., Kundur, A.R., and Singh, I. 2020. Anthocyanin supplementation inhibits secretion of
pro-inflammatory cytokines in overweight and obese individuals. Journal of Functional Foods. 64: 103596.
Wei, J. Chen, J-R., Pais, E.M.A., WangT-Y., Miao, L., Li, L., Li, L-Y., Qiu, F., Hu, L-M., Gao, X-M. et al. 2017. Oxyresveratrol is a phytoestrogen exerting anti-inflammatory effects through NF-kappaB and estrogen receptor signaling. Inflammation. 40(4): 1285-1296.
Wongon, M. and Limpeanchob, N. 2020. Inhibitory effect of Artocarpus lakoocha Roxb. and oxyresveratrol on α-glucosidase and sugar digestion in Caco-2 cells. Heliyon. 6(3): e03458.
Yang, P., Zhao, Y., Zhao, L., Yuan, J., Chen, Y., Varghese, Z., Moorhead, J.F., Chen, Y., and Ruan, X.Z. 2015. Paradoxical effect of rapamycin on inflammatory stress-induced insulin resistance in vitro and in vivo. Scientific Reports. 5: 14959.
OPEN access freely available online
Natural and Life Sciences Communications
Chiang Mai University, Thailand. https://cmuj.cmu.ac.th
Saowalak Yomlar, Kanittaporn Trisat, and Nanteetip Limpeanchob*
Department of Pharmacy Practice, Pharmacological Research Unit, Faculty of Pharmaceutical Sciences and Center of Excellence for Innovation in Chemistry, Naresuan University, Phitsanulok, 65000 Thailand
Corresponding author: Nanteetip Limpeanchob, E-mail: nanteetipl@nu.ac.th
Total Article Views
Editor: Wipawadee Yooin,
Chiang Mai University, Thailand
Article history:
Received: June 3, 2023;
Revised: October 30, 2023;
Accepted: November 29, 2023;
Online First: December 12, 2023

