
Development of a Palm Kernel-Based Emulgel Loaded with Mitracarpus scarber Leaf Extracts for Effective Anti-Inflammatory Activity
Zwanden Sule Yahaya*, Ummar Adamu, Uwaisu Iliyasu, Oluwaseun Adenike Orugun, Fatima Shuibu Kurfi, and Charles Na’anman DagogotPublished Date : November 23, 2023
DOI : https://doi.org/10.12982/NLSC.2024.001
Journal Issues : Number 1, January-March 2024
Abstract
Mitracarpus scaber is a tropical herb used in ethnomedicine for the treatment of inflammation and a variety of other diseases. The aim of this study was to develop a palm kernel-based emulgel containing Mitracarpus scarber and examine the product's physicochemical characteristics and anti-inflammatory efficacy on Wister rats. Different batches of emulgel comprising Mitracarpus scarber leaf extracts (MSE), different proportions of oils (palm kernel oil and/or liquid paraffin), and surfactants (tween-80 and/or Cremophor EL-30) were prepared. The freshly prepared and 5-month-old formulations, stored at room temperature (28 ± 2 °C), were evaluated for rheology, syneresis, and extract/excipient compatibility using Fourier transform infrared (FT-IR) spectroscopy. The spreadability, extrudability, and anti-inflammatory activity of the optimal formulation were compared with those of a commercial anti-inflammatory emulgel. Even after five months of storage, the created emulgels remained olive green and creamy, with a homogeneous texture, consistency, and glossy appearance. The optimized batch containing palm kernel oil, tween-80, and xanthan gum had the most stable characteristics, with no significant change in pH (P = 1.000) or FT-IR spectrum after 5 months of storage. It exhibited spreadability and extrudability of 12.75 cm2 and 600 g/cm2, respectively, compared to 16.82 cm2 and 400 g/cm2 for the commercial formulation. Four hours post-treatment, the anti-inflammatory effect of the optimized batch was significantly (P < 0.05) higher than that of the aqueous MSE dispersion. The obtained results demonstrate the prospects of palm kernel-based emulgel loaded with Mitracarpus scarber leaf extracts as an innovative therapeutic approach to inflammation treatment.
Keywords: Inflammation, Emulgel, Mitracarpus scarber, Palm kernel oil, Formulation
Citation: Yahaya, Z.S., Adamu, U., Iliyasu, U., Orugun, O.A., Kurfi, F.S., and Dagogot, C.N. 2024. Development of a palm kernel-based emulgel loaded with Mitracarpus scarber leaf extracts for effective anti-inflammatory activity. Natural and Life Sciences Communications. 23(1): e2024001.
INTRODUCTION
An injury, an infection, or irritation is what causes the body to experience inflammation as a defense mechanism. Redness, swelling, heat, pain, and occasionally loss of function in the affected area are its most prominent features (Ashley et al., 2012). Inflammation occurs when the immune system detects damaged or foreign cells in the body and sends white blood cells to the affected site. These white blood cells produce substances that stimulate blood arteries to dilate, allowing more blood to flow to the affected area. The redness and warmth linked to inflammation are brought on by this increased blood flow. Additionally, the white blood cells produce substances that lead to blood vessel fluid leakage into the surrounding tissue, resulting in swelling and discomfort (Varela et al., 2018; Roe, 2021). Inflammation can be treated in a variety of ways. Among these is the use of nonsteroidal anti-inflammatory drugs (NSAIDs) or corticosteroids (Sweetman, 2009; Maslikah et al., 2023). Changes in lifestyle, such as eating healthy meals, exercising regularly, and lowering stress, can all help to minimize chronic inflammation (Furman et al., 2019; Margină et al., 2020). Omega-3 fatty acids, ginger, and other organic remedies such as turmeric may also aid to relieve inflammation (Sears, 2015).
Mitracarpus scaber Zucc. (Rubiaceae) is a tropical herb that is native to Nigeria, Ghana, Gambia, and Senegal. The herb is widely used in traditional medicine in Nigeria to cure a variety of diseases (Ekpendu et al., 1994). Pre-clinical findings also demonstrate that the plant is useful against a number of diseases, including anti-inflammatory benefits (Akah and Nwambie, 1994; Audoin et al., 2018; Francis Obiora et al., 2019; Fawehinmi and Oyedeji, 2020; Abizi et al., 2021; Ekalu, 2021). Reports suggest that palm kernels may have anti-inflammatory characteristics due to their high levels of lauric acid, tocotrienols, carotenoids, and pyroligneous acid, all of which have been shown to possess anti-inflammatory effects (Huang et al., 2014; Nainggolan and Sinaga, 2021; Rabiu et al., 2021). The aim of this study was to develop a palm kernel-based emulgel containing Mitracarpus scarber and examine the product's physicochemical characteristics and anti-inflammatory efficacy on Wister rats. Emulgels are formed by incorporating an emulsion system into a gel matrix (Sharma et al., 2018). Emulgels function as a dual control release system since the emulsion and the gel matrix are both control release systems. When emulgels are employed in the topical delivery of medicaments, they release the drugs from the inner phase (emulsion phase) to the outer phase (gel phase) onto the skin in a controlled manner (Ali Khan et al., 2020; Okpalaku et al., 2022). Most herbal extracts are hydrophobic, and emulgels are among the delivery techniques that have been effective in addressing some of the biopharmaceutical difficulties related to the administration of hydrophobic drug molecules (Culbertson et al., 1976; Ashara et al., 2016; Ali Khan et al., 2020). Emulgels have the added benefit of protecting the active moiety from hydrolysis and enzymatic degradation, as well as a higher spreading coefficient than most semisolids (Okpalaku et al., 2022). Emulgels are also greaseless, thixotropic, soothing, non-staining, easily removable, and compatible with a wide range of excipients (Ashara et al., 2016; Ali Khan et al., 2020).
MATERIALS AND METHODS
Plant material collection and preparation
Fresh Mitracarpus scarber leaves were collected in December 2021 from Mayare, Makarfi Local Government Area, Kaduna State (North-west Nigeria), identified and assigned the voucher number KASU/BSH/1212 at the Department of Biological Science, Kaduna State University. After 14 days of air drying, the leaves were pulverized into a powder with a clean mortar and pestle. The powdered leaves were macerated in 70% ethanol for 6 days at room temperature while being agitated every 12 hours. After filtering the extract using a muslin cloth, the filtrate was concentrated using a rotary evaporator, and the percentage yield was calculated. The resultant Mitrcarpus scarber extract (MSE) was stored in an airtight container until usage.
Qualitative phytochemical screening
Following standard procedures (Sofowara, 1993; Harborne, 1998; Evans, 2002), the crude extract was tested for alkaloids, saponins, flavonoids, glycosides, polysaccharides, tannins, anthraquinones, and terpenoids.
MSE emulgel preparation
The formulations made and the different amounts of components utilized are shown in Table 1. In order to form the gel, xanthan gum was mixed with hot distilled water and repeatedly shaken at a moderate speed with a mechanical shaker until the liquid had a homogeneous consistency. Distilled water, an ethanolic menthol solution, methyl parabens dissolved in propylene glycol, and the MSE dissolved in ethanol were combined to produce the emulsion's aqueous phase. While the oil phase of the emulsion was made by dissolving the surfactant (Tween 80 and/or Cremophor EL-35) in the oil (palm kernel oil and/or liquid paraffin). Both the oily and aqueous phases were heated to a temperature of 70 – 80 °C before the oily phase was added dropwise to the aqueous phase and continuously stirred for 30 minutes until it cooled to room temperature. To make the emulgel, the resulting emulsion was mixed with the xanthan gel in a 1:1 ratio with moderate stirring for 30 minutes (Ali Khan et al., 2020).
Table 1. MSE emulgel formulations’ quantitative compositions (% w/w).
|
Ingredients |
A1 |
A2 |
A3 |
A4 |
|
MSE |
0.50 |
0.50 |
0.50 |
0.50 |
|
Xanthan gum |
5.69 |
5.69 |
5.69 |
5.69 |
|
Palm kernel oil |
2.00 |
2.00 |
2.00 |
1.00 |
|
Liquid paraffin |
- |
- |
- |
1.00 |
|
Tween-80 |
0.5 |
0.25 |
- |
0.25 |
|
Cremophor EL-35 |
- |
0.25 |
0.50 |
0.25 |
|
Propylene glycol |
0.65 |
0.65 |
0.65 |
0.65 |
|
Ethanol |
0.78 |
0.78 |
0.78 |
0.78 |
|
Methyl parabens |
0.01 |
0.01 |
0.01 |
0.01 |
|
Menthol |
1.25 |
1.25 |
1.25 |
1.25 |
|
Purified water to |
25.00 |
25.00 |
25.00 |
25.00 |
MSE emulgel organoleptic properties assessment
The color, odor, after-feel, consistency, phase separation and appearance of the developed formulations were all visually inspected (Vanpariya et al., 2021). This was repeated on samples that had been stored for 5 months at room temperature (28 ± 2 °C).
Rheological evaluation
The viscosity of the formulated emulgels was measured using rotor no. 2 of a rotary viscometer (NDJ-SS Digital viscometer, SearchTech, instrument, London, United Kingdom). The measurements were taken at ambient temperature (28 ± 2 °C). The viscometer readings were obtained once they were seen to be stable for 2 - 3 seconds. This was repeated on the samples after 5 months of storage at room temperature (28 ± 2 °C).
Syneresis measurement
Exactly 3 g of the formulated emulgel was weighed and transferred into a perforated-bottom cylindrical plastic tube with a foil paper covering. The tube and its contents were centrifuged for 15 minutes, after which the weight of the tube and the liquid that had been separated were measured, and Equation 1 was used to compute the percentage syneresis (Ashara et al., 2016):

pH determination
The prepared emulgels' pH was measured using a digital pH meter by submerging the glass electrode in the emulgel for 1 minute and recording the pH. The pH values of each formulation were measured and recorded (Rahil et al., 2015). This was carried out again on the samples that had been stored for 5 months at room temperature (28 ± 2 °C).
Compatibility test
Fourier Transform Infrared (FTIR) spectra of freshly prepared and 5-month-old formulations were obtained to examine the formulation's characteristics. The spectra were collected between 4000 and 650 cm-1 (Ali Khan et al., 2020).
Optimization criteria and further evaluation
The preliminary examination of the formulated batches revealed that A1 had the most stable characteristics (no significant change in FTIR after 5 months of storage), hence it was selected as the optimized batch.
Spreadability measurement
After sandwiching the optimized formulation between two glass slides, a 200 g weight was placed on top and left for 10 seconds. Equation 2 was used to compute the area of spread (Ilievska et al., 2016). This was compared with the spreadability of a commercial product.
Area = length x breadth (2)
Extrudability measurement
Equation 3 was used to calculate the amount of emulgel (cm2) that could be extruded from a collapsible tube with a 400 g weight (Ilievska et al., 2016).

Anti-inflammatory assessment
Wistar rats of both sexes weighing 150 - 200 g were obtained from the Department of Pharmacology's animal house facilities at Ahmadu Bello University in Zaria, Nigeria. The animals were kept under natural ventilation at a temperature of 28 ± 2 °C, with access to water ad libitum, and were fed rat pellets from Vital Feeds Limited in Ibadan, Nigeria. The experimental methods were carried out in accordance with the criteria approved by the Ahmadu Bello University Committee on Animal Use and Care (ABUCAUC/2018/017). Using the approach described by Yahaya et al., (2022) the formulations' anti-inflammatory effects were examined. This study employed five groups of five animals (mature Wistar rats of either sex weighing 150-200 g). Group I received 0.9% w/v normal saline; Group II received a 1% aqueous MSE dispersion; Group III received the developed emulgel base; Group IV received the formulated MSE emulgel (1%); and Group V received the marketed emulgel (1%). To induce edema, 0.1 ml of fresh, undiluted egg albumin was injected sub-plantar. Thirty minutes before the egg yolk injection, the treatment (as previously described) was gently applied over the plantar surface of the left hind paw of the rat. The thickness of the paw was measured using a Vernier caliper at 0, 1, 2, 3, 4, 5, and 6 hours after the egg albumin injection. The percentage inhibition of paw edema was calculated using Equation 4.

Et = Average edema of the group
Ec = Average edema of the Control Group
Statistical analysis
IBM SPSS Inc. software (version 23), Chicago, Illinois, USA, was used for the statistical analysis. One-way ANOVA and the Bonferroni post hoc test were used to compare the means of different groups. The results were considered significant at P < 0.05.
RESULTS
Phytochemical screening, organoleptic, rheological, syneresis, pH determination, and compatibility test
The MSE yield was 19.14%. The result of the qualitative phytochemical screening is presented in Table 2. The formulated emulgels were olive green, creamy, viscous, and opaque, with a homogenous texture and glossy appearance, as shown in Figure 1. Table 3 shows the syneresis, viscosity, and pH of all batches when newly produced and after 5 months of storage at room temperature (28 ± 2 °C). The pH results ranged between 4.0 and 5.3. The FTIR spectrum of MSE revealed a strong sharp band at 1028.7 cm-1 (-C-O stretching vibration), a medium sharp band at 1580.4 cm-1 (-C=C aromatic ring stretch), 2922.2 cm-1 (aliphatic -C-H stretching vibration), and a broad band at 3302.4 cm-1 (-OH group). The spectra of the different batches of MSE emulgel, in addition to the aforementioned peaks, exhibited medium sharp bands in the range of 1636.3 cm-1 – 1744.0 cm-1 (carbonyl groups) and 1148.0 cm-1 – 1155.0 cm-1 (asymmetric vibration of the phosphate (PO2) group). The FTIR spectra are shown in Figure 2. The optimized formulation had spreadability and extrudability of 12.75 cm2 and 600 g/cm2, respectively, compared to 16.82 cm2 and 400 g/cm2 for the commercial formulation.
Table 2. Phytochemical constituents of MSE.
|
Phytochemical group |
Qualitative assay |
|
Carbohydrate |
+ |
|
Saponins |
+ |
|
Flavonoids |
+ |
|
Tannins |
+ |
|
Terpenoids |
+ |
|
Alkaloids |
- |
|
Anthraquinones |
+ |
|
Glycosides |
+ |

Figure 1. Physical appearance of the formulation.
Table 3. Some physicochemical characteristics of the different batches when freshly prepared and after 5 months of storage at ambient temperature (28 ± 2 °C).
|
Test |
Formulation |
Freshly prepared |
5-month old formulation |
P - value |
|
Viscosity (mPa s) |
A1 |
4.06 ± 0.12 |
5.39± 0.24 |
0.000* |
|
A2 |
4.00 ± 0.13 |
3.95± 0.08 |
1.000 |
|
|
A3 |
3.35 ± 0.15 |
4.07 ± 0.07 |
0.103 |
|
|
A4 |
4.04 ± 0.04 |
4.01 ± 0.04 |
1.000 |
|
|
Syneresis (%) |
A1 |
35.7 ± 0.25 |
48.0 ± 2.10 |
0.000* |
|
A2 |
35.0 ± 0.00 |
49.0 ± 4.30 |
0.000* |
|
|
A3 |
22.7 ± 0.90 |
29.3 ± 1.40 |
0.014* |
|
|
A4 |
12.0 ± 0.20 |
17.7 ± 1.30 |
0.047* |
|
|
pH |
A1 |
4.1 ± 0.17 |
4.1 ± 0.40 |
1.000 |
|
A2 |
5.2 ± 0.10 |
4.8 ± 0.20 |
1.000 |
|
|
A3 |
4.0 ± 0.30 |
4.3 ± 0.10 |
1.000 |
|
|
A4 |
5.3 ± 0.10 |
5.3 ± 0.20 |
1.000 |

Figure 2. Fourier-transform infrared spectra of MSE, freshly prepared and 5-month old Batch A1, A2, A3, and A4
Anti-inflammatory properties
The rats' hind paws exhibited a substantial inflammatory response after receiving an intraplantar injection of egg albumin, which led to paw edema. One hour following the injection of egg albumin, rats in all the groups started to exhibit observable changes in the gross morphology of their paws, including redness and swelling. The edema in the paws peaked at 3 hours, as seen in Table 4, and then began to decline. Figure 3 shows the anti-inflammatory characteristics of the different formulations in terms of preventing paw edema.
Table 4. Mean increase in rats’ paws 1 to 6 hours after egg albumin injection.
|
Treatment |
Mean increase in paw edema (mm) ± SD |
|||||
|
1h |
2h |
3h |
4h |
5h |
6h |
|
|
Control |
3.42 ± 0.22 |
3.65 ± 1.05 |
3.44 ± 1.13 |
2.11 ± 0.96 |
1.55 ± 0.59 |
2.31 ± 0.53 |
|
Aqueous MSE dispersion |
2.81 ± 0.42 |
3.18 ± 0.85 |
3.04 ± 0.48 |
1.88 ± 0.24 |
1.41 ± 0.08 |
0.98 ± 0.04 |
|
Emulgel base (placebo) |
3.07 ± 0.43 |
2.67 ± 0.33 |
2.92 ± 0.38 |
1.77 ± 0.28 |
1.13 ± 0.05 |
1.07 ± 0.20 |
|
Formulated MSE emulgel |
2.34 ± 0.23 |
2.97 ± 0.66 |
1.93 ± 0.66 |
1.04 ± 0.04 |
0.80 ± 0.64 |
0.61 ± 0.45 |
|
Marketed emulgel |
2.61 ± 0.44 |
2.85 ± 1.14 |
3.13 ± 0.67 |
1.70 ± 0.69 |
1.06 ± 0.35 |
1.05 ± 0.32 |
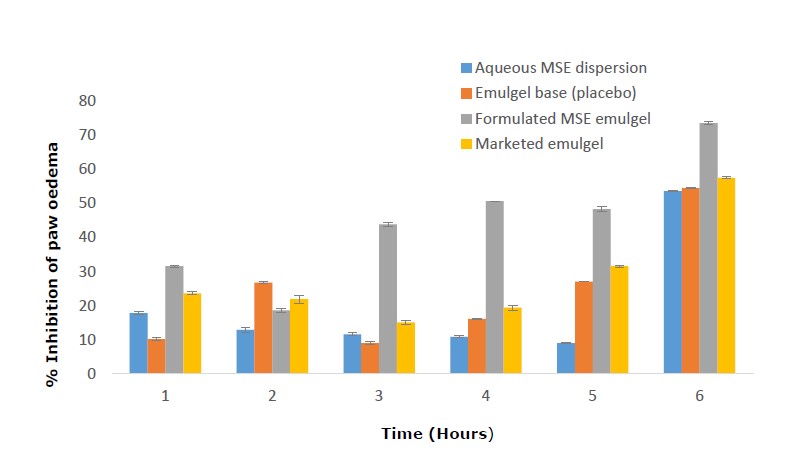
Figure 3. Inhibition percentage of edema in rats’ paws by the various preparations 1 to 6 hours after egg albumin injection.
DISCUSSION
Saponins, flavonoids, glycosides, carbohydrates, tannins, anthraquinones, and terpenoids were found in the phytochemical screening, while alkaloids were absent. There have been reports of most of these biomolecules having anti-inflammatory activities, particularly saponins, flavonoids, glycosides, tannins, and terpenoids (Mohammed et al., 2014; Oguntibeju, 2018; Khelfi et al., 2023).
Elegance and visual appeal have a beneficial impact on product acceptability and, ultimately, marketing success (Ilievska et al., 2016; Arab et al., 2022). The emulgels kept their color, homogeneity, consistency, and appearance five months after formulation. Given that uniformity of the dosage form and drug content release depends significantly on viscosity, viscosity is a crucial characteristic to be assessed (Ali Khan et al., 2020). Batch A1 was the most viscous, both the freshly prepared and after 5 months of storage. This is due to the higher viscosity of the oil (palm kernel) and surfactant (tween-80) content compared to the other batches, which contained less viscous liquid paraffin (oil) and Cremophor EL-35 (surfactant). Syneresis is the removal or expulsion of a liquid from a gel (Arab et al., 2022). The gel system of an emulgel often shrinks after standing; this occurrence, known as syneresis, can cause a little amount of liquid to be squeezed out of a product, reducing its visual appeal. Syneresis measurement is a technique for estimating liquid leaks from products (Mizrahi, 2010). After 5 months of storage, the obtained syneresis results of all batches increased significantly (P < 0.05). Measures such as package design, keeping the product at a stable temperature as much as possible, and preventing external pressure can reduce or even prevent syneresis (Mizrahi, 2010). There were no significant (P = 1.000) differences in the pH of the batches after 5 months of storage. Changes in the pH of a product could thus be a result of product instability (Ali Khan et al., 2020).
The compatibility of the extracts/drugs, and excipients employed in the preparation of pharmaceutical dosage forms can be evaluated via FTIR spectral analysis (Burki et al., 2020). Chemical interaction or degradation can cause changes in spectral shape, which might reflect changes in a sample's overall content (Yahaya et al., 2023). All the peaks of MSE were present in the freshly prepared formulation. After 5 months of storage, however, significant changes were observed when the spectra of freshly prepared and 5-month-old formulations were compared for batches A2, A3, and A4. This ranges from the reduction of the intensity of the hydroxyl group to the increase of the aliphatic -C-H stretching vibration (for batch A2), shifting of the C-O stretching vibration, and disappearance of the aliphatic -C-H stretching vibration (for batch A3). In batch A4, the C-O stretching vibration shifted the hydroxyl group band to a higher frequency. In batch A1, however, there was no observable significant difference between the peaks of the freshly prepared and 5-month-old formulations. Within the scope of our work, we can deduce that the optimal outcomes for employing palm kernel oil as a lipophilic carrier in emulgel formulations are obtained when it is used in combination with tween-80 as the surfactant and xanthan gum as the gelling agent.
Spreadability refers to how easily a semisolid formulation spreads over a surface (Dantas et al., 2016; Ilievska et al., 2016). The shear required to push or expel a semisolid formulation from a tube or container, on the other hand, is referred to as extrudability (Naga Sravan et al., 2014; Ilievska et al., 2016). The ease with which semisolid products can be withdrawn and applied depends on both of these properties. It follows, therefore, that while more shear will be needed to extract the optimized formulation from its tube or container than the commercial product, it will spread more readily. Emulgels must be easily spreadable in order to be used topically since semisolids' capacity to spread makes them better suited for topical administration. Furthermore, it is thought that this has a substantial impact on how successfully patients adhere to their medications (Dantas et al., 2016). Six hours post-treatment, the anti-inflammatory effects of the different preparations were in the following order: aqueous MSE dispersion < emulgel base (placebo) < marketed emulgel < MSE emulgel. The anti-inflammatory activity of MSE was markedly enhanced by formulating it as an emulgel. Four hours after treatment, the anti-inflammatory effect of MSE emulgel was significantly (P < 0.05) higher than that of aqueous MSE dispersion, which could be attributed to the emulgel's ability to promote efficient and prolonged access of the product to the skin and facilitate penetration and delivery of the MSE. The emulgel base displayed anti-inflammatory activity comparable to the aqueous MSE dispersion, lending credence to reports by Huang et al., (2014); Nainggolan and Sinaga, (2021) and Rabiu et al., (2021) that palm kernels have anti-inflammatory properties. As a result, the MSE, palm kernel oil, and other formulation excipients appear to have collaborated to achieve the improved anti-inflammatory effect demonstrated by the MSE emulgels.
CONCLUSION
A palm kernel-based emulgel loaded with Mitracarpus scarber leaf extracts produced improved anti-inflammatory effects in rats compared to the aqueous dispersion of the extract. Phytochemical analysis revealed several important biomolecules in the extract that might be responsible for the anti-inflammatory activity. The batch containing palm kernel oil, Tween-80, and xanthan gum had the most stable characteristics, with no significant change in FT-IR spectrum after 5 months of storage. The formulation's improved performance offers a better strategy and scientific basis for the development of Mitracarpus scarber leaf extracts for the management of inflammation.
ACKNOWLEDGMENTS
The authors are thankful to the laboratory staff of the animal house facility of the Department of Pharmacology, Ahmadu Bello University Zaria, as well as those of Kaduna State University's Department of Pharmaceutics and Industrial Pharmacy, for their technical assistance and for providing the necessary infrastructural and laboratory facilities.
AUTHOR CONTRIBUTIONS
Concept design and development: Zwanden Sule Yahaya, Uwaisu Iliyasu and Na’anman Charles Dagogot
Data collection analysis and interpretation - Ummar Adamu, Oluwaseun Adenike Orugun and Fatima Shuibu Kurfi
Literature search and manuscript drafting - Zwanden Sule Yahaya, Ummar Adamu, Oluwaseun Adenike Orugun and Fatima Shuibu Kurfi
Final manuscript review and approval - Zwanden Sule Yahaya, Uwaisu Iliyasu, Charles Na’anman Dagogot, Ummar Adamu, Oluwaseun Adenike Orugun and Fatima Shuibu Kurfi.
CONFLICT OF INTEREST
The authors declare that they hold no competing interests.
REFERENCES
Abizi, G.O.S., Fatou, S.E., Zougrou N’guessan, J., Kouadio, K.E., Begbin, K.S., Koffi, J.J., Kablan, K., and Koné, A. 2021. Anti-arthritic activity of the aqueous extract of the aerial parts of Mitracarpus scaber (Rubiaceae) Zucc in rats Wistar. Journal of Diseases and Medicinal Plants. 7(1): 22.
Akah, P.A., and Nwambie, A.I. 1994. Evaluation of Nigerian traditional medicines: 1. Plants used for rheumatic (inflammatory) disorders. Journal of Ethnopharmacology. 42: 179-I82.
Ali Khan, B.S., Ullah, M.K., Khan, S.M., Alshahrani, and Braga, V.A. 2020. Formulation and evaluation of Ocimum basilicum-based emulgel for wound healing using animal model. Saudi Pharmaceutical Journal. 28(12): 1842–1850.
Arab, M.M., Yousefi, E., Khanniri, M., Azari, V., Ghasemzadeh, M., and Mollakhalili-Meybodi, N. 2022. A comprehensive review on yogurt syneresis: Effect of processing conditions and added additives. Journal of Food Science and Technology. 19(9): 33.
Ashara, K.C., Paun, J.S., Soniwala, M.M. Chavda, J.R. Mendapara, V.P.and Mori, N.M. 2016. Microemulgel: An overwhelming approach to improve therapeutic action of drug moiety. Saudi Pharmaceutical Journal, 24(4): 452–457.
Ashley, N.T., Weil, Z.M. and Nelson, R.J. 2012. Inflammation: Mechanisms, costs, and natural variation. Annual Review of Ecology, Evolution, and Systematics. 43(1): 385–406.
Audoin, C.A., Zampalégré, N. Blanchet, A. Giuliani, E. Roulland, O. Laprévote, K., and Genta-Jouve, G. 2018. MS/MS-guided isolation of clarinoside, a new anti-inflammatory pentalogin derivative. Molecules. 23(5): 1237.
Burki, I.K., M.K., Khan, B.A., Khan, B., Uzair, V.A., and Jamil. Q.A. 2020. Formulation Development, characterization, and evaluation of a novel dexibuprofen-capsaicin skin emulgel with improved in vivo anti-inflammatory and analgesic effects. AAPS PharmSciTech. 21(6): 211.
Culbertson, M.R., Donahue, T.F., and Henry, S.A. 1976. Control of inositol biosynthesis in Saccharomyces cerevisiae: Properties of a repressible enzyme system in extracts of wild-type (Ino+) cells. Journal of Bacteriology. 126(1): 232–242.
Dantas, M.G.B., Reis, S.A.G.B., Damasceno, C.M.D., Rolim, L.A., Rolim-Neto, P. J., Carvalho, F.O., Quintans-Junior, L.J., and Almeida, J.R.G.D.S. 2016. Development and evaluation of stability of a gel formulation containing the monoterpene borneol. The Scientific World Journal. 2016:7394685 (1–4).
Ekalu, A. 2021. Medicinal uses, phytochemistry, and pharmacological activities of Mitracarpus species (Rubiaceae): A review. Scientific African. 11: e00692.
Ekpendu, T.O., Akah, P. A., Adesomoju, A. A., and Okogun, J.I. 1994. Antiinflammatory and antimicrobial activities of Mitracarpus scaber extracts. International Journal of Pharmacognosy. 32(2): 191–196.
Evans, W.C. 2002. Trease and Evans Pharmacognosy. Habid, W.B Saunders, Edinburgh, United Kindom.
Fawehinmi, A.B., and Oyedeji, F.O. 2020. Evaluation of formulated anti-dermatophyte creams from ethanol extract of Mitracarpus villosus Leaves. International Journal of Biochemistry Research and Review. 29(9) 1–12.
Francis, O.O., Bege, J., Joel, A.I., and Barnabas, N.J. 2019. Crude extracts of Mitracarpus scaber roots significantly ameliorate paracetamol (PCM)-induced liver damage in rats. American Journal of Biomedical and Life Sciences. 7(60): 148.
Furman, D., Campisi, J., Verdin, E., Carrera-Bastos, P., Targ, S. 2019, Chronic inflammation in the etiology of disease across the life span. Nature Medicine. 25(12): 1822–1832.
Harborne, J. B., 1998. Phytochemical methods. A guide to modern techniques of plant analysis. Chapman and Hall, London.
Huang, W.C., Tsai, T.H., Chuang, L.T., Li, Y.Y., Zouboulis, C.C., and Tsai, P.J. 2014, Anti-bacterial and anti-inflammatory properties of capric acid against Propionibacterium acnes: A comparative study with lauric acid. Journal of Dermatological Science. 73(3): 232–240.
Ilievska, B., Loftsson, T. Hjalmarsdottir, M., and Asgrimsdottir, G. 2016. Topical formulation comprising fatty acid extract from cod liver oil: Development, evaluation and stability studies. Marine Drugs. 14 (6): 105.
Khelfi, S., Zerizer, S., Foughalia, A., Tebibel, S., Bensouici, C., and Kabouche, Z. 2023. The antioxidant activity and the anti-inflammatory effect of Citrus sinensis L. fruit on intestinal inflammation induced by hyperhomocysteinemia in mice. Natural and Life Sciences Communications. 22(1): e2023009.
Margină, D., Ungurianu, A., Purdel, C., Tsoukalas, D., Sarandi, E. 2020. Chronic inflammation in the context of everyday life: Dietary changes as mitigating factors. International Journal of Environmental Research and Public Health. 17(11): 4135.
Maslikah, S.I., Lestari, S.R., Handayani, N., Putra, W.E., Alimah, A.R.N., Amalia, A., Afifah, S., and Arifah, S.N. 2023. The anti-inflammatory potential of red betel (Piper crocatum) leaves through inhibitory mechanism on NFΚB signaling pathway: Drug-like candidate study. Natural and Life Sciences Communications. 22(1): e2023005.
Mizrahi, S. 2010. Syneresis in food gels and its implications for food quality. p.324–348. In S. Mizrahi (ed) Chemical Deterioration and Physical Instability of Food and Beverages. Elsevier, London.
Mohammed, M.S., Osman, W.J.A., Garelnabi, E.A.E. Osman, Z., Osman, B., Khalid, H.S., and Mohamed, M.A. 2014. Secondary metabolites as anti-inflammatory agents. The Journal of Phytopharmacology 3(4): 275–285.
Naga Sravan, K.V., Maheshwari, V.P.V., Navya, M., Reddy, S.C., Shivakumar, H.G., and Gowda, D.V. 2014. Calcipotriol delivery into the skin as emulgel for effective permeation. Saudi Pharmaceutical Journal. 22(6): 591–599.
Nainggolan, M., and Sinaga, A. S. 2021. Characteristics of fatty acid composition and minor constituents of red palm olein and palm kernel oil combination. Journal of Advanced Pharmaceutical Technology and Research. 12(1): 22.
Oguntibeju, O., 2018. Medicinal plants with anti-inflammatory activities from selected countries and regions of Africa. Journal of Inflammation Research. 11: 307–317.
Okpalaku, O., Uronnachi, E., Okoye, E., Umeyor, C., Nwakile, C., Okeke, T., and A. Attama, 2022. Evaluating some essential oils-based and coconut oil nanoemulgels for the management of rheumatoid arthritis. Letters in Applied NanoBioScience. 12(3): 75.
Rabiu, Z., Hamzah, M.A., Hasham, R., and Zakaria, Z.A. 2021. Characterization and antiinflammatory properties of fractionated pyroligneous acid from palm kernel shell. Environmental Science and Pollution Research. 28(30): 40535–40543.
Rahil, G.M., Khushboo, A.B., and Shah, S.K. 2015. Formulation and evaluation of topical nano emulgel of adapalene. World Journal of Pharmaceutical Sciences. 3(4): 1013–1024.
Roe, K., 2021. An inflammation classification system using cytokine parameters. Scandinavian Journal of Immunology. 93(20): 1232.
Sears, B., 2015. Anti-inflammatory diets. Journal of the American College of Nutrition. 34: 14–21.
Sharma, V., Nayak, S.K., Paul, S.R., Choudhary, B., Ray, S.S. and Pal, K. 2018. Emulgels. p. 251–264. In V. Sharma (ed) Polymeric Gels: Elsevier, London.
Sofowara, A. 1993. Screening plants for bioactive agents. Medicinal Plants and Traditional Medicinal in Africa. Spectrum Books Ltd, Sunshine House, Ibadan, Nigeria.
Sweetman, S.C. 2009. Martindale: The complete drug reference. Pharmaceuticale Press, PhP, London.
Vanpariya, F., Shiroya, M., and Malaviya, M. 2021. Emulgel: A review. International Journal of Science and Research. 10(3): 847–852.
Varela, M. L., M. Mogildea, I. Moreno, and Lopes, A. 2018. Acute inflammation and metabolism. Inflammation. 41(4): 1115–1127.
Yahaya, Z.S., Giwa, L., Dagogot, N. Orugun, O.A., Adeleye and Mohammed, B.B. 2023. Evaluation of the suspending properties of Parkia biglobosa mucilage in a metronidazole suspension formulation. American Journal of Pharmacotherapy and Pharmaceutical Sciences. 2(4): 1-8.
Yahaya, Z.S., Kurfi, F.S. Mallam, D., Oloyede, R.B., Adeleye, O.A., and Okpanachi, G.O. 2022. Preliminary research on ibuprofen self-emulsifying formulation. Journal of Research in Pharmacy. 26(4): 834–841.
OPEN access freely available online
Natural and Life Sciences Communications
Chiang Mai University, Thailand. https://cmuj.cmu.ac.th
Zwanden Sule Yahaya1, *, Ummar Adamu1, Uwaisu Iliyasu2, Oluwaseun Adenike Orugun1, Fatima Shuibu Kurfi1, and Charles Na’anman Dagogot3
1 Department of Pharmaceutics and Industrial Pharmacy, Faculty of Pharmaceutical Sciences, Kaduna State University, Kaduna, Nigeria.
2 Department of Pharmacognosy and Drug Development, Faculty of Pharmaceutical Sciences, Kaduna State University, Kaduna, Nigeria.
3 Department of Pharmaceutics and Industrial Pharmacy, Faculty of Pharmaceutical Sciences, Ahmadu Bello University, Zaria, Nigeria.
Corresponding author: Zwanden Sule Yahaya, E-mail: zwanden.yahaya@kasu.edu.ng
Total Article Views
Editor: Nisit Kittipongpatana,
Chiang Mai University, Thailand
Article history:
Received: August 18, 2023;
Revised: October 28, 2023;
Accepted: November 14, 2023;
Online First: November 23, 2023

