
Design and Optimization of Ganciclovir Nanosuspension Loaded In Situ Gelling Mucoadhesive Eye Drops for Herpetic Keratitis
Phuvamin Suriyaamporn, Boonnada Pamornpathomkul, Theerasak Rojanarata, Prasopchai Patrojanasophon, Praneet Opanasopit, and Tanasait Ngawhirunpat*Published Date : October 31, 2023
DOI : https://doi.org/10.12982/NLSC.2023.068
Journal Issues : Number 4, October-December 2023
Abstract Ganciclovir (GCR), an antiviral drug used to treat herpetic keratitis, is less effective because of its poor bioavailability (BCS III). Nanosuspension (NS) is a promising technique for improving solubility and the dissolution of poorly soluble drugs by size reduction. Topical solution or conventional eye drop represents the easiest route to deliver drugs to the anterior eye segment; however, low precorneal retention, high nasolacrimal drainage, and metabolic degradation lead to low bioavailability. To overcome these limitations, incorporating GCR-NS into mucoadhesive in situ gel-forming (ISG) could enhance its ocular permeability and drug bioavailability. Therefore, this study aimed to design and optimize GCR-NS-loaded in situ gelling mucoadhesive eye drops (GCR-NS-loaded mucoadhesive ISG) for improving ophthalmic delivery. Pluronic® F127 (P127), an ophthalmic gel forming, was selected as a polymer due to self-gelling formation by temperature-triggered in situ gelling at ocular temperature. Moreover, hyaluronic acid-modified catechol (HA-cat), a novel mucoadhesive polymer, was combined to achieve the desired ocular mucoadhesion and facilitate ophthalmic delivery. Optimized GCR-NS formulation prepared using the nanoprecipitation technique was selected based on the central composite design (CCD) model by evaluating their particle size, polydispersity index (PDI), and zeta potential, before being loaded into mucoadhesive ISG. The optimal GCR-NS-loaded mucoadhesive ISG (F7) revealed the desired physicochemical properties with better viscosity, mucoadhesion, and gelling capacity at physiological conditions, high ocular permeation with a sustained manner over 24 h compared to eye drop suspension. Accordingly, GCR-NS-loaded mucoadhesive ISG could be a promising ocular delivery system for the effective local delivery of GCR for herpetic keratitis.
Keywords: Ganciclovir, Nanosuspension, Mucoadhesive eye drop, Ophthalmic delivery, In situ gelling
Citation: Suriyaamporn, P., Pamornpathomkul, B., Rojanarata, T., Patrojanasophon, P., Opanasopit, P., and Ngawhirunpat, T. 2023. Design and optimization of ganciclovir nanosuspension loaded in situ gelling mucoadhesive eye drops for herpetic keratitis. Natural and Life Sciences Communications. 22(4): e2023068.
INTRODUCTION
Herpes simplex virus (HSV) is an important pathogen leading to herpetic keratitis, the corneal viral infection. Herpetic keratitis is a cause of severe infections that can lead to scarring of the cornea or human blindness problem worldwide. The global herpetic keratitis incidence is approximately 1.5 million annually (Pandey et al., 2020). The severity of HSV infection can be categorized according to the depth of the ocular tissue layers: epithelial keratitis, stromal keratitis, and endothelial keratitis. Approximately 80% of HSV-infected ocular tissue is acute epithelial keratitis (Chou et al., 2014). General symptoms were found, including phengophobia, ophthalmodynia, tearing, signs of ciliary congestion, corneal infiltration/ulcer, and corneal edema. Risk factors of HSV infection are exposure to ultraviolet radiation, use and abuse of corticosteroids, trauma, systemic diseases, and patients who use immunosuppressive drugs (Ahmad et al., 2023; Tabbara et al., 2010). HSV can be divided into two types by the location of infection. HSV type 1 specifically infects ocular and orofacial, while HSV type 2 generally causes genital disease. However, HSV-2 can also infect ocular tissues in the neonatal when the mother has a genital HSV-2 infection (genital to ocular infection) (Ahmad et al., 2023; Liesegang, 2001).
The Herpes Eye Disease Study (HEDS) suggested topical formulations containing antiviral agents as the front-line treatment for herpetic keratitis, approved by the Ocular Microbiology and Immunology Group (White et al., 2014). In contrast, using oral antiviral drugs at high doses to reach the target site results in unfavorable systemic toxicities (Ahmad et al., 2023; Farooq et al., 2012). Therefore, topical eye drop formulation is the most preferred route for delivering therapeutic agents to the anterior eye segment as it benefits from non-invasion, reducing systemic side effects, avoiding first-pass metabolism, reducing drug dosage administration, and increasing patient compliance (Gote et al., 2019). Recently, topical antiviral agents that have been approved in the United States and Europe for herpetic keratitis treatment are idoxuridine, iododesoxycytidine, vidarabine, 1% trifluridine (Viroptic), 3% acyclovir (Zovirax®), and 0.15% ganciclovir ophthalmic gel (Zirgan™) (Ahmad et al., 2023; Naito et al., 1987).
Ganciclovir (GCR), a synthetic acyclic nucleoside analog of 2’-deoxyguanosine, is a broad-spectrum antiviral with activity against both HSV-1 and HSV-2 at relatively low inhibitory concentrations (IC50 of ∼50 ng/mL) (Sohail Akhter et al., 2013). GCR is transformed into triphosphate-GCR (TP-GCR) in virus-infected cells by cellular kinases before it inhibits the viral DNA replication process (competitively binding to DNA polymerase) (Lin et al., 2013). Therefore, it is safer than other topical antiviral agents because it selectively targets viral DNA, resulting in less toxicity to normal cells and a favorable tolerability profile (Chou et al., 2014; Hoh et al., 1996). The conventional treatment of GCR, oral administration at a high dose regimen of 3 g/day for herpetic keratitis treatment, is associated with serious side effects such as bone marrow suppression and neutropenia. Therefore, GCR topical treatment is preferred (S. Akhter et al., 2012; Chou et al., 2014). However, the GCR topical formulation had a low permeability, categorized in Biopharmaceutics Classification System (BCS) Class III with a water solubility of approximately 2-3 mg/mL at 25 °C (Parr et al., 2016). Therefore, frequent administration is necessary because of their short retention time caused by high tear-fluid turnover rates, high nasolacrimal drainage, and corneal barrier. To overcome these problems, novel ocular drug delivery systems such as nanoparticle, nanosuspension, in situ gel-forming, and mucoadhesive polymers have been developed to enhance precoroneal residence and bioavailability of the therapeutic agents (Fangueiro et al., 2016).
Nanosuspension (NS), a colloidal dispersion of nanosized particles, shows a state of matter characterized by higher surface area, solubility, and dissolution rate leading to increase ocular permeation and bioavailability (Kassem et al., 2007; Pınar et al., 2023). The NS can be prepared by milling, high-pressure homogenization, and precipitation techniques. Among them, the precipitation technique or bottom-up process is a simple preparation and requires less energy than the others. Hence, it is the most currently used in the process of NS preparation, in which the drug is solved in an organic solvent and then precipitated in an anti-solvent to form a very fine amorphous or crystalline nanosized particle (Soltani et al., 2016). Several NS formulations have been successfully developed and used for topical ocular drug delivery. However, NS formulations could not provide suitable retention time and prolong drug release to achieve bioavailability. Moreover, topical NS formulation might be easily eliminated by blinking or nasolacrimal drainage (Gupta et al., 2013; Suriyaamporn et al., 2021; Purva Khare et al., 2022). Combining NS with in situ gelling mucoadhesive formulation has recently increased interest in ocular drug delivery (Szalai et al., 2022).
In situ gelling (ISG) with mucoadhesive properties could effectively increase retention time and improve drug bioavailability (Szalai et al., 2022). The ISG ophthalmic formulation is typically applied in the ocular tissue as a solution state (sol), resulting in the strong gel forming through a phase transition by various mechanisms with nonchemical cross-linking such as temperature-sensitive mechanism (poloxamers (Cook et al., 2022)), cellulose derivates (Arvidson et al., 2013)), a pH-responsive mechanism (carbomers (Al-Kinani et al., 2018)), and ion-activated mechanism (gellan gum (Elmowafy et al., 2019)). Pluronic® F127 (Poloxamer 407 or P127) has been widely used to prepare ophthalmic ISG because of the formation of self-gelling by temperature-triggered in-situ gelling at ocular temperature. P127 is a non-ionic biocompatible and biodegradable polymer suitable for suspending the drug's small and large molecules. The temperature-sensitive polymer was selected to develop an ocular ISG formulation because it can easily modify the composition by adjusting the temperature and controlling drug release. The P127 can form the in-situ gelling at ocular temperature (31 to 37 °C) (Cao et al., 2010; K. A. Soliman et al., 2019; Shah et al., 2021; Abbas et al., 2022).
Hyaluronic acid (HA), a natural polysaccharide, has a wide range of eye applications, such as dry eye treatment, vitreous substitutes, and ophthalmic viscosurgical devices. It has excellent biocompatibility and biodegradability (Zhang et al., 2021). However, using unmodified hyaluronic acid (HA) in ocular drug delivery is challenging due to its poor mucoadhesive properties. To improve the limitation, modifications targeting the amine, thiol, and catechol functional groups on the HA structure have been explored to enhance its mucoadhesive properties (Kim et al., 2021). A novel mucoadhesive polymer, hyaluronic acid catechol (HA-cat), incorporating functional modifications, was combined with a thermosensitive polymer to enhance mucoadhesion. This new-generation mucoadhesive polymer can establish permanent covalent bonds with mucin glycoproteins in ocular tissues. Catechol (Cat) functional groups, known for their adhesive properties in underwater mussel adhesion, have been investigated as multi-modal mucoadhesion side chains in glycoproteins. Consequently, the interaction between the new-generation mucoadhesive polymer and mucin glycoproteins in ocular tissues can prolong the precorneal residence time, thereby improving the bioavailability of the drug (Pornpitchanarong et al., 2020).
This study aimed to design and optimize the GCR-NS-loaded ISG mucoadhesive eye drops using temperature-triggered in situ gelling polymer for ophthalmic delivery. The NS was formulated by precipitation technique, and the optimized formulation was then selected from the lowest particle size, PDI, and the suitable zeta potential using statistic computer-aided design (central composite design model). The physicochemical properties of GCR-NS-loaded ISG mucoadhesive eye drops were determined. In vitro mucoadhesion and permeation through ocular tissues were performed.
MATERIALS AND METHODS
Materials
Ganciclovir (GCR) was purchased from the Tokyo Chemical Industry (Tokyo, Japan). Polyvinyl alcohol (PVA; 99+% hydrolyzed; molecular weight 89-98 kDa), sodium lauryl sulfate (SLS), dopamine hydrochloride, N-(3-dimethylaminopropyl)-N’-ethylcarbodiimide hydrochloride (EDAC), and N-hydroxysuccinimide (NHS) were purchased from Sigma Aldrich (Dorset, United Kingdom). Pluronic® F127 (P127; MW = 4,000 Da) was supplied by BASF chemicals company (Ludwigshafen, Germany). Hyaluronic acid (HA; 1200–1800 kDa) was obtained from P.C. Drug Center (Bangkok, Thailand). All other chemicals and solvents used were analytical grades.
Preparation and optimization of GCR-NS by DoE
Design of experiment (DoE) is a part of the Quality by Design (QbD) approach that helps to understand not only the effect of variables but also the interactions between them (Fukuda et al., 2018). The effects of independent variables, such as the amount of PVA, SLS, and deionized water (DI water), were selected according to a central composite design model (CCD). The dependent variables were particle size, PDI, and zeta potential. The response surface method (RSM) and regression equation were generated to explain the relationship between the independent and dependent variables. The total of 19 experiments that fit the models and optimization criteria of the suitable formulation is presented in Table 1. The results of the experiments were analyzed using Design Expert® 11 computer software (Stat-Ease, Minneapolis, USA). The confidence interval was 95%, and the p-value indicating a significant variable was 0.05. The insignificant coefficients (P-value > 0.05) were eliminated from the regression equations to increase the fitting accuracy and coefficient of determination values (R2).
Table 1. Variables values of the CCD for GCR-NS.
|
Independent variables |
Range |
||||
|
- α |
-1 |
0 |
+1 |
+α |
|
|
X1: Amount of PVA (mg) |
98.87 |
150 |
225 |
300 |
351.13 |
|
X2: Amount of SLS (mg) |
1.59 |
5 |
10 |
15 |
18.41 |
|
X3: Volume of DI water (mL) |
1.59 |
5 |
10 |
15 |
18.41 |
|
Dependent variables |
Optimization |
||||
|
Y1: Particle size |
Minimize |
||||
|
Y2: PDI |
Minimize |
||||
|
Y3: Zeta potential |
In target (> -20 mV) |
||||
The GCR-NS was prepared by a bottom-up technique (anti-solvent nanoprecipitation technique). An accurately weighed GCR (75 mg) was taken and completely dissolved in 50 mL of DMSO. Several clinical trials have repoorted that 0.15-0.20% GCR topical ophthalmic gel was safe and effective in the treatment of herpetic keratitis (Chou et al., 2014). In the anti-solvent beaker, PVA, SLS, and DI water were mixed at various amounts according to the DoE, as shown in Table 2. The 0.1% benzalkonium chloride as a preservative was added to the anti-solvent beaker. To precipitate GCR particles, the drug solution was slowly dropped (0.15 mL/min) into the anti-solvent beaker using an infusion pump with a syringe connected to needle gauge no.22 while stirring at 500 rpm and maintained the temperature at 4 ± 1 °C. To reduce the particle size, probe sonication (VCX 500, Sonics Vibra-Cell, USA) was used at an amplitude of 40% (125 W, 20 kHz) for 30 min. The GCR-NS formulations were stirred overnight at room temperature. Finally, GCR-NS formulations were dialyzed using dialysis membrane (MW cut-off 3.5 kDa, Thermo Fisher Scientific Inc., Illinois, USA) against DI water to remove the organic solvent and excess stabilizers. Fresh DI water was replaced every 6 h for 3 cycles. GCR-NS was obtained and kept at 4°C until use (Jakubowska et al., 2022; N. Wang et al., 2021).
Table 2. Compositions of GCR-NS-loaded ISG mucoadhesive formulations.
|
Formulation codes |
% |
% Pluronic® F127 |
% HA |
% HA-cat |
% Benzalkonium chloride |
Water q.s. to (mL) |
|
F1 |
0.15% |
5.00% |
0.10% |
- |
0.10% |
100 |
|
F2 |
0.15% |
5.00% |
0.50% |
- |
0.10% |
100 |
|
F3 |
0.15% |
5.00% |
- |
0.10% |
0.10% |
100 |
|
F4 |
0.15% |
5.00% |
- |
0.50% |
0.10% |
100 |
|
F5 |
0.15% |
10.00% |
0.10% |
- |
0.10% |
100 |
|
F6 |
0.15% |
10.00% |
0.50% |
- |
0.10% |
100 |
|
F7 |
0.15% |
10.00% |
- |
0.10% |
0.10% |
100 |
|
F8 |
0.15% |
10.00% |
- |
0.50% |
0.10% |
100 |
|
F9 |
0.15% |
15.00% |
0.10% |
- |
0.10% |
100 |
|
F10 |
0.15% |
15.00% |
0.50% |
- |
0.10% |
100 |
|
F11 |
0.15% |
15.00% |
- |
0.10% |
0.10% |
100 |
|
F12 |
0.15% |
15.00% |
- |
0.50% |
0.10% |
100 |
Characterizations of GCR-NS
Morphology of GCR-NS
The morphological attributes of GCR powder and GCR-NS were analyzed with a transmission electron microscope (TEM) (Philips TECNAI 20, UK). The samples were diluted with DI water (1:10), dropped on a carbon film-coated copper grid, and counter-stained with 2% phosphotungstic acid for 3 min, then dried under a fume hood at room temperature for 24 h. After that, the samples were accelerated at a voltage of 100 kV. Photomicrographs were visualized at appropriate magnifications (Kuk et al., 2019).
Particle size, PDI, and zeta potential
Particle size, PDI, and zeta potential of GCR-NS were determined using a dynamic light scattering analyzer (Zetasizer Nano Series, Malvern Instruments, Malvern, UK). The system was set at 25 °C, 90° angle. Before analysis, the GCR-NS was diluted with DI water (1:10) and filled into a disposable cell. The samples were analyzed in triplicate.
Synthesis of catechol-functionalized hyaluronic acid (HA-CAT)
The HA functionalized with catechol was synthesized following the previous study (Pornpitchanarong et al., 2020). Pornpitchanarong and coworkers reported that the highest degree of substitution (DS) for HA-CAT was achieved when using HA and dopamine at a weight ratio of 1:3, yielding a result of around 0.95. Briefly, 0.5%w/v HA was dissolved in a round-bottom flask containing 100 mL of DI water under magnetic stirring until a clear solution was obtained. Then, EDAC and NHS, equal to the molar quantity of dopamine, were slowly added to the reaction flask. Following 30 min, 0.75 g of dopamine hydrochloride was added, and the pH was adjusted to reach 4.5-5.5. The reaction was continued under a nitrogen atmosphere and dark place for 18 h. After the reaction was complete, HA-cat was subsequently dialysis using a dialysis membrane (MW cut-off 3.5 kDa, Thermo Fisher Scientific Inc., Illinois, USA) against acidified PBS (pH 4.5-5.5) for 3 cycles (6 h/cycle) followed by DI water (pH 4.5-5.5) for 1 cycle to remove uncoupled dopamine hydrochloride. Finally, the HA-cat was lyophilized and kept at 4 °C until use.
Preparation of GCR-NS-loaded ISG mucoadhesive eye drop
Different GCR-NS-loaded ISG mucoadhesive eye drop formulations were prepared, as shown in Table 2. To obtain GCR-NS-loaded ISG mucoadhesive eye drops, the thermosensitive ocular gel, Pluronic® F127 (P127) was prepared by the modified cold method. Firstly, P127 was dissolved in the DI water while stirring in an iced bath at 4 °C. Next, various concentrations of HA and HA-cat, mucoadhesive polymers, were slowly added into the polymer solution at 4 °C. The samples were refrigerated overnight to achieve a clear homogeneous solution. Finally, the optimized GCR-NS formulation was added to each mixture and mixed until a homogeneous solution was obtained. The GCR-NS-loaded ISG mucoadhesive formulations were subjected to terminal sterilization in an autoclave at 118 °C for 5 min under a pressure of 15 psi (Beard et al., 2020; Galante et al., 2018; Haridas et al., 2019; Khare et al., 2022; Zielińska et al., 2020).
Characterizations of GCR-NS-loaded ISG mucoadhesive eye drops
Clarity
The clarity of GCR-NS-loaded ISG mucoadhesive formulations was observed by visual examination against white and black backgrounds under the light before and after gelling. The clarity of DI water, ISG mucoadhesive formulations, and GCR-NS-loaded ISG mucoadhesive formulations were compared (Baranowski et al., 2014; Gupta et al., 2007).
Gelling capacity
The gelling capacity of prepared GCR-NS-loaded ISG mucoadhesive formulations was determined using simulated tear fluid (STF; pH 7.4). STF (pH 7.4) was prepared by dissolving NaCl (6.78 g/L), NaHCO3 (2.18 g/L), CaCl2 • 2H2O (0.084 g/L), and KCl (1.38 g/L) in DI water. The samples (100 µL) were dropped into the vial containing 2 mL of STF at 37 °C. The time required for gelling was observed and recorded (Baranowski et al., 2014).
pH value
The pH was one of the most important parameters in ophthalmic formulations because it affected drug solubility and stability and may cause eye irritation. The pH value of optimal GCR-NS and GCR-NS-loaded ISG mucoadhesive formulations in colloid form were directly determined using calibrated pH meter (Laquatwin Horiba, Kyoto, Japan) (Khare et al., 2022).
Determination of drug content
The drug content of GCR loaded in GCR-NS-loaded ISG mucoadhesive formulations was determined using HPLC. The sample was dissolved and appropriately diluted in acetonitrile (ACN). After that, the sample was filtered through a 0.45 µm nylon syringe filter before analysis. GCR content was analyzed using HPLC (Agilent, Santa Clara, CA, USA) equipped with a reversed-phase C8 column (4.6 x 150 mm, 5 μm) (Zorbax eclipse, Agilent, USA). The mobile phase was a mixture of 2%v/v ACN: 98%v/v 0.05 M ammonium acetate (pH 2.5), a 1 mL/min flow rate, and a detection wavelength of 254 nm. The volume injected was 25 μL using the autoinjector. The column temperature was set at 35 °C. (Merodio et al., 2000). The percentage of drug loading was calculated following Equation 1.

Viscosity and rheology
The viscosity properties of GCR-NS-loaded ISG mucoadhesive formulations were measured using a Brookfield DV2T (Toronto, Canada). Each formulation (7 mL) was poured into a small chamber assembly connected with a spindle SC4-13RP at a controlled temperature of 37.0 ± 0.5°C. The viscosity of the samples was measured based on a percentage torque value observed between 80-90% at a spindle speed of 100 rpm. The centipoise (cP) of each sample was recorded.
To evaluate the rheological behavior of optimal GCR-NS-loaded ISG mucoadhesive formulation, the rheometer (DSR Malvern–Kinexus Pro, USA) was connected to a plate-plate type measuring device (PP25-SN 17002; diameter 40 mm; gap height 0.50 mm). The gelling temperature of the optimal formulation was determined by increasing the temperature from 4 °C to 50 °C with a constant heating rate of approximately 3 °C/min. The measurement was determined at a constant shear rate of 1 rad/min and a constant strain of 1%. The temperature point of the sol-gel transition was observed at the crossover of the storage modulus (G′) and the loss modulus (G″) curves.
Mucoadhesive property
The mucoadhesive property was measured with a texture analyzer (TA.XT plus, Stable Microsystems, Godalming, UK) connected with a 5 kg load cell. A mucoadhesion test rig was fixed with corneal tissue in the tissue holder. STF (100 µL, pH 7.4) was dropped on the corneal surface to represent a human’s tear. The mucoadhesion test rig was submerged in a beaker filled with water at 37 °C. 0.15% GCR-NS and GCR-NS-loaded ISG formulations (5 mL) were added to the corneal surface. The cylinder probe (5 mm diameter) was fitted into the hole of the tissue holder, which contacted the corneal surface. The probe was slowly lowered to the corneal surface at a speed of 0.5 mm/s and a 2.5 N preload was used for 1 min. Then the probe was moved up at the same speed until the adhesive bond was broken. The adhesive force (N) was calculated to determine the mucoadhesive behavior of the formulations (Amorós et al., 2022; Szalai et al., 2022).
In vitro release study and kinetics
In vitro release study and kinetics of GCR suspension, GCR-NS, GCR-NS-loaded ISG formulation, and GCR-NS-loaded ISG mucoadhesive formulation were determined using a dialysis method. The dialysis bag with a molecular weight cut-off of 6,000 (Cellu Sep® T2, Texas, USA) was used. The dialysis bags containing 2 mL of each sample were immersed in 75 mL PBS (pH 7.4), and the temperature was controlled at 37±0.5°C while being constantly shaken at 150 rpm. At predetermined times (0, 30 min, 1, 2, 4, 6, 8, 12, and 24 h), 1 mL of the release medium was withdrawn and replaced with an equivalent volume of the new medium. The release medium was analyzed and reported as a percentage of cumulative GCR release versus times. The GCR release graph was fitted to various mathematical models, including zero order, first order, Higuchi, Korsmeyer-Peppas, and Hixson-Crowell, to illustrate the release mechanism. The best fit model, indicated by the highest R2 value, was selected (Suriyaamporn et al., 2023).
In vitro corneal permeation study
The transcorneal permeability of GCR was evaluated using a porcine eye cornea. The fresh whole porcine eyeballs were obtained from the local slaughterhouse (Nakhon Pathom Province, Thailand) and kept in PBS (pH 7.4) at 4 °C until used (within 4 h). The porcine cornea with 2-4 mm of surrounding sclera tissue was carefully excised using surgical scissors and then soaked in PBS at 37 °C for 30 min before use. The excised porcine cornea with epithelial surface upward was fixed between a donor and receptor compartment of a vertical Franz diffusion cell. The receptor compartment was filled with freshly prepared PBS (pH 7.4). The temperature was controlled at 37 ± 0.5 °C and continuously stirred with a magnetic stirrer at 50 rpm. The 0.5 mL of GCR suspension, GCR-NS, GCR-NS-loaded ISG formulation, and GCR-NS-loaded ISG mucoadhesive formulation were separately added into the donor compartment. At predetermined times (15 min, 30 min, 1 h, 2 h, 4 h, 6 h, 8 h, and 24 h), the 500 μL of each sample from the receptor compartment was withdrawn, an equal volume of fresh PBS was replaced to maintain a constant volume. The concentrations of GCR permeated from different formulations were analyzed by HPLC (Majeed et al., 2019).
The remaining GCR in porcine cornea
After completing the in vitro corneal permeation study, the porcine cornea was removed and cleaned with fresh PBS (pH 7.4). The porcine cornea was sliced into small pieces and homogenized using a probe sonicator in 20 mL of PBS (pH 7.4) at 60 Hz for 20 min. The extracted sample was centrifuged at 4,000×g for 10 min to precipitate the corneal tissues. The supernatant was collected and filtered by a 0.45 µm nylon syringe filter before being analyzed by HPLC (Suriyaamporn et al., 2022).
Statistical analysis
The statistical analysis of the central composite design was performed by Design Expert® 11 computer statistic software (Stat-Ease, Minneapolis, USA). The descriptive results are presented as mean ± SD in triplicate. Where appropriate, in vitro experiments were analyzed using Student’s t-test and one-way analysis of variance (ANOVA) with Tukey’s HSD post hoc test. A value of P < 0.05 was considered statistically significant.
RESULTS
Preparation and optimization of GCR-NS by DoE
GCR-NS was successfully prepared using a nanoprecipitation technique. The particle size, PDI, and zeta potential of GCR-NS were measured, as shown in Table 3. To determine the best composition of GCR-NS, 19 formulations were created according to the central composite design matrix by varying the concentrations of PVA (X1), SLS (X2), and antisolvent (X3). The particle size of GCR-NS ranged from 146.70 to 1928.40 nm, with a PDI of 0.24 to 1.00, and the zeta potential ranged from -2.70 to -29.40 mV. A multiple regression analysis was used to identify the most appropriate regression function explaining the relationship between independent and dependent variables, with coefficients estimated based on a p-value of less than 0.05. In addition, the lack-of-fit test was used to ensure that the estimated model’s mean value of repeated measurements was non-significant (P-value > 0.05).
Table 3. Central composite design representing experimental runs with independent and dependent variables.
|
Formulation codes |
Independent variables (X) |
Dependent variables (Y) |
||||
|
X1: PVA(mg) |
X2: SLS(mg) |
X3: Antisolvent (mL) |
Y1: Size(nm) |
Y2: PDI |
Y3: Zeta potential (mV) |
|
|
NS1 |
98.87 |
10.00 |
10.00 |
220.00 |
0.24 |
-16.50 |
|
NS2 |
150.00 |
5.00 |
5.00 |
146.70 |
0.57 |
-13.80 |
|
NS3 |
150.00 |
5.00 |
15.00 |
993.00 |
0.53 |
-24.00 |
|
NS4 |
150.00 |
15.00 |
5.00 |
735.70 |
0.56 |
-15.30 |
|
NS5 |
150.00 |
15.00 |
15.00 |
351.10 |
0.57 |
-29.40 |
|
NS6 |
225.00 |
1.59 |
10.00 |
521.80 |
0.87 |
-2.70 |
|
NS7 |
225.00 |
10.00 |
1.59 |
312.10 |
0.83 |
-12.10 |
|
NS8 |
225.00 |
10.00 |
10.00 |
492.70 |
0.63 |
-7.70 |
|
NS9 |
225.00 |
10.00 |
10.00 |
268.00 |
0.36 |
-8.50 |
|
NS10 |
225.00 |
10.00 |
10.00 |
354.20 |
0.45 |
-10.30 |
|
NS11 |
225.00 |
10.00 |
10.00 |
245.10 |
0.34 |
-7.20 |
|
NS12 |
225.00 |
10.00 |
10.00 |
409.20 |
0.34 |
-8.30 |
|
NS13 |
225.00 |
10.00 |
18.41 |
133.10 |
0.65 |
-12.40 |
|
NS14 |
225.00 |
18.41 |
10.00 |
345.60 |
0.70 |
-8.30 |
|
NS15 |
300.00 |
5.00 |
5.00 |
1444.50 |
1.00 |
-11.50 |
|
NS16 |
300.00 |
5.00 |
15.00 |
1355.50 |
0.30 |
-23.00 |
|
NS17 |
300.00 |
15.00 |
5.00 |
881.60 |
0.56 |
-10.80 |
|
NS18 |
300.00 |
15.00 |
15.00 |
629.20 |
1.00 |
-10.20 |
|
NS19 |
351.13 |
10.00 |
10.00 |
1928.40 |
0.96 |
-15.90 |
Table 4 presents the ANOVA and multiple regression analysis results for each response obtained from the CCD model. The P-values for all responses were less than 0.05, indicating that the quadratic model was significant in explaining the relationship between the independent and dependent variables. The P-values of the lack-of-fit test were greater than 0.05, indicating that the quadratic model was suitable for all responses except for Y3 (zeta potential). The regression coefficients (R2) of Y1 (particle size) and Y2 (PDI) were 0.8657 and 0.7468, respectively. R2 value greater than 0.7-0.8 was typically considered appropriate for a model (Moore et al., 2013). Therefore, the R2 of Y3 (zeta potential) at around 0.3283 was deemed inappropriate for optimizing the GCR-NS formulation.
Table 4. Summary of the ANOVA and multiple regression analysis for CCD.
|
Responses |
|
Fit Summary |
||||
|
Model |
P-value |
Lack of fit |
R2 |
Predicted R2 |
Recommended |
|
|
Particle size |
Quadratic |
< 0.0500 |
0.0646 |
0.8657 |
0.6493 |
Suggested |
|
PDI |
Quadratic |
0.0045 |
0.3952 |
0.7468 |
0.6202 |
Suggested |
|
Zeta potential |
Quadratic |
0.0478 |
< 0.0500 |
0.3283 |
-0.2174 |
Not recommended |
|
Multiple regression equation models (coded equation) |
||||||
|
Particle size (Y1) = 393.40 + 393.29X1 – 90.68X2 -204.54X1X2 + 285.06X12 |
||||||
|
PDI (Y2) = 0.47 + 0.16X1 – 0.02X2 – 0.02X3 + 0.11X2X3 + 0.11X22 + 0.09X32 |
||||||
|
Zeta potential (Y3) = –10.44 + 2.05X1 – 3.63X12 |
||||||
The appropriate criteria were analyzed to optimize the GCR-NS, as shown in Table 5. The optimal GCR-NS formulation was the combination of 150.00 mg of PVA, 8.845 mg of SLS, and 11.37 mL of anti-solvent with a desirability of 0.91. Student’s t-test statistical analysis was used to assess the bias of the selected model by comparing the predicted results (Rp) and actual results (Ra). The confirmation showed that the model chosen was reliable, as there was no significant difference between Rp and Ra (Table 5).
Table 5. Optimization and confirmation of GCR-NS formulation.
|
Optimization |
|||
|
Variables |
Criteria |
Optimized results |
Desirability |
|
PVA (mg) |
in range |
150.000 mg |
0.910 |
|
SLS (mg) |
in range |
8.845 mg |
|
|
Anti-solvent (mL) |
in range |
11.37 mL |
|
|
Particle size |
Minimize |
259.853 nm |
|
|
PDI |
Minimize |
0.323 |
|
|
Zeta potential |
none |
-20.120 mV |
|
|
Confirmation |
|||
|
Results |
Size (nm) |
PDI |
Zeta potential (mV) |
|
Rp |
259.52 ± 0.13 |
0.51 ± 0.01 |
-16.12 ± 0.00 |
|
Ra |
259.53 ± 70.25 |
0.36 ± 0.08 |
-21.43 ± 6.60 |
|
T-test (p-value) |
1.00 |
0.51 |
0.30 |
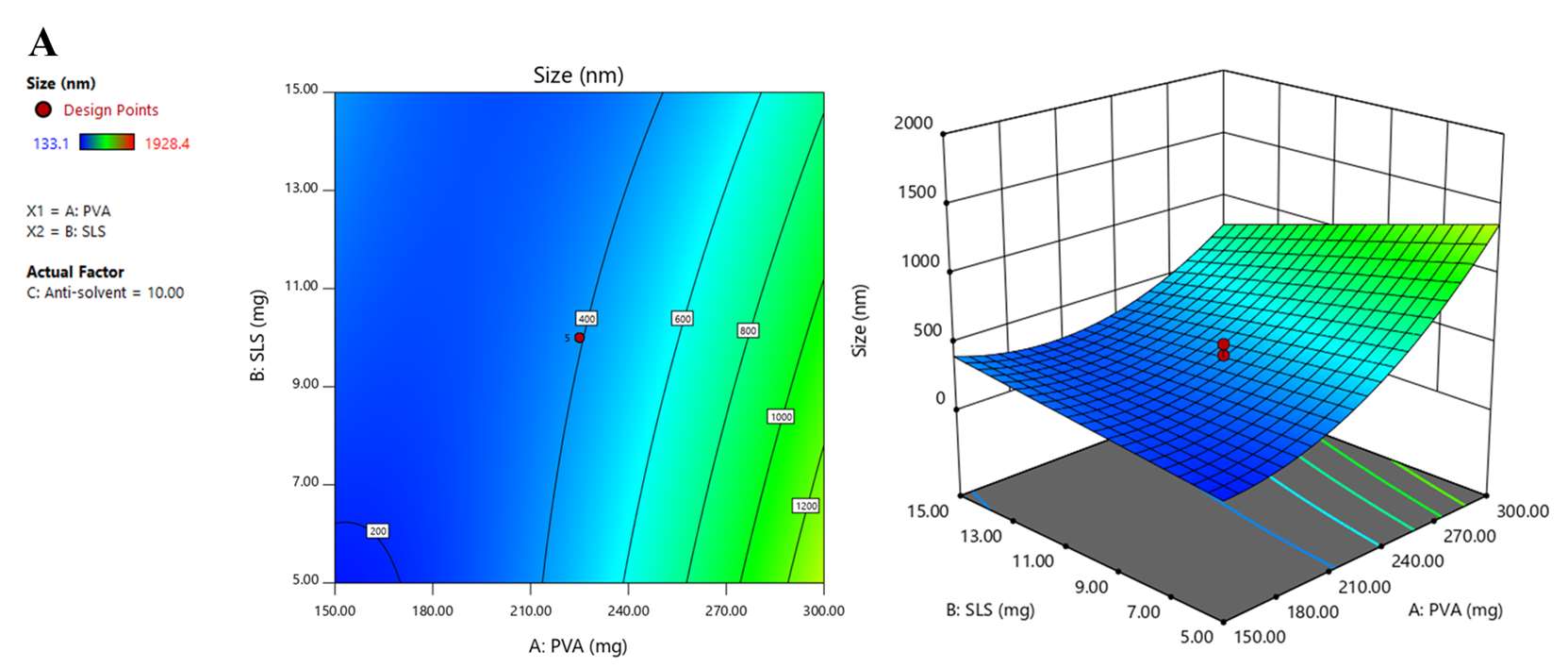
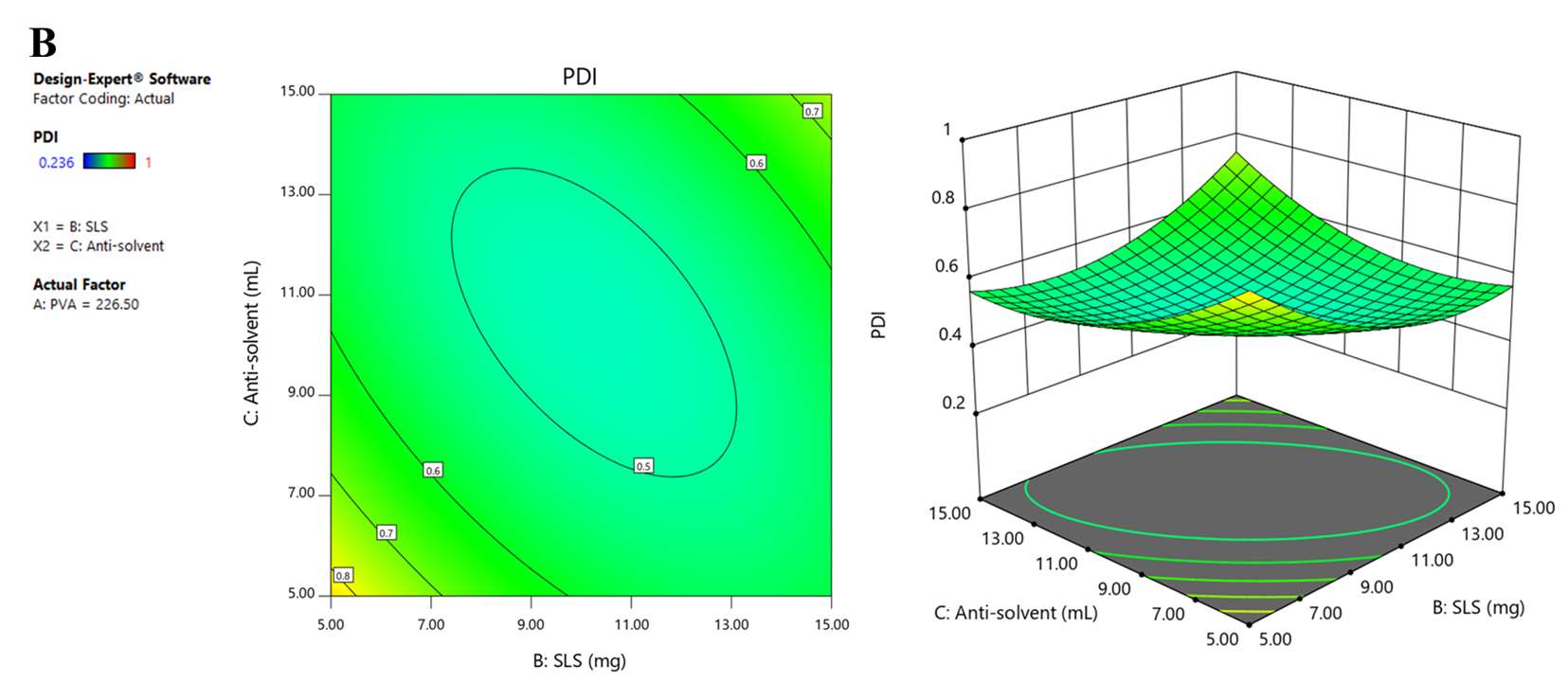

Figure 1. 2D and 3D response surface graphs demonstrating the influence of the formulation and process parameters on particle size (A), PDI (B), and zeta potential (C).
Morphology of GCR-NS
TEM images presented in Figure 2 showed coarse GCR powder and GCR-NS. The mean particle size of the NS in the optimized formula was around 300 nm, consistent with the results in Table 5. Meanwhile, the coarse GCR powder had a particle size of about 1.5 µm. TEM images of GCR-NS demonstrated that the particle size was spherical and smaller than coarse GCR powder.
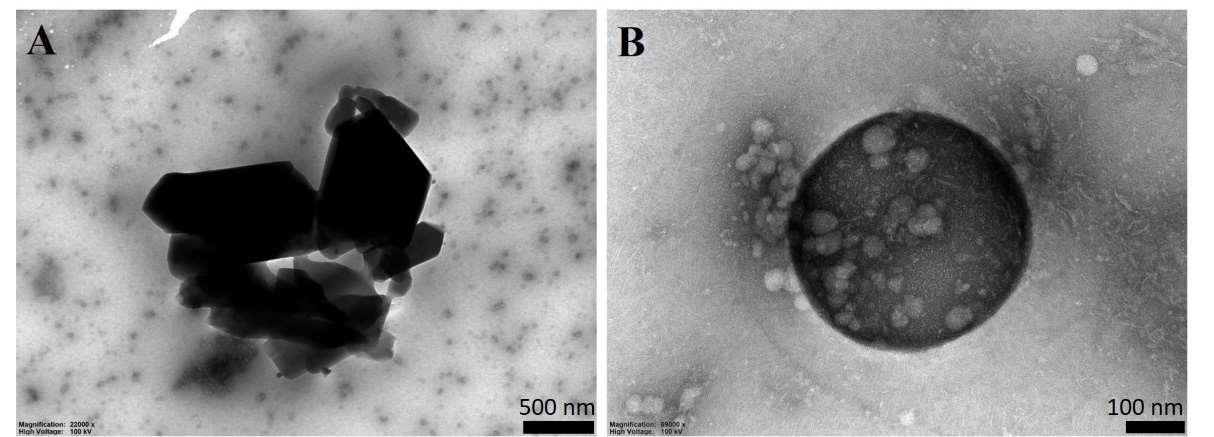
Figure 2. TEM images of coarse GCR powder (A) and GCR-NS (B)
Characterizations of GCR-NS-loaded ISG mucoadhesive eye drops
The physicochemical evaluation of ISG mucoadhesive eye drops was conducted to determine their clarity, gelling capacity, pH, and drug content. All ISG formulations were clear before and after gelling, as presented in Table 6. The GCR-NS-loaded ISG revealed immediate gelling capacity upon exposure to simulated tear fluid within around 20 sec at 37 °C, and the formed gels remained stable for an extended period (24 h). The pH of all formulations ranged around 5.3-6.7. The percentage drug loading (%DL) of GCR-NS in ISG ranged from 96.04% to 99.85%.
Table 6. Physicochemical properties of GCR-NS-loaded ISG mucoadhesive eye drops (mean ± SD; n = 3).
|
Formulations |
Clarity |
Gel capacity (sec) |
pH |
%Drug content |
Viscosity (cP) |
Adhesive force (N) |
|
F1 |
Clear |
21.60 ± 1.00 |
6.20 ± 0.20 |
96.70 ± 1.30 |
189.8 0± 10.40 |
3.30 ± 0.20 |
|
F2 |
Clear |
20.90 ± 0.10 |
6.40 ± 0.30 |
96.15 ± 0.05 |
1944.00 ± 23.10 |
4.50 ± 1.20 |
|
F3 |
Clear |
19.10 ± 0.20 |
6.30 ± 0.10 |
97.31 ± 0.26 |
21.70 ± 1.90 |
8.30 ± 3.40 |
|
F4 |
Clear |
18.90 ± 2.00 |
5.90 ± 0.10 |
96.89 ± 2.40 |
190.60 ± 15.00 |
10.20 ± 2.10 |
|
F5 |
Clear |
22.80 ± 1.10 |
6.50 ± 0.20 |
99.61 ± 0.42 |
92.60 ± 7.10 |
6.40 ± 1.50 |
|
F6 |
Clear |
22.20 ± 1.00 |
6.60 ± 0.40 |
97.11 ± 0.12 |
3435.00 ± 92.20 |
9.30 ± 2.40 |
|
F7 |
Clear |
16.30 ± 1.20 |
6.50 ± 0.20 |
99.85 ± 0.62 |
72.07 ± 2.50 |
14.30 ± 2.10 |
|
F8 |
Clear |
15.50 ± 0.50 |
5.50 ± 0.10 |
98.13 ± 1.22 |
557.40 ± 5.80 |
15.30 ± 3.50 |
|
F9 |
Clear |
12.10 ± 0.10 |
6.60 ± 0.30 |
96.04 ± 2.01 |
402.40 ± 20.50 |
15.90 ± 1.20 |
|
F10 |
Clear |
10.30 ± 0.40 |
6.70 ± 0.20 |
97.11 ± 1.26 |
5040.40 ± 98.20 |
23.40 ±3.20 |
|
F11 |
Clear |
10.00 ± 0.50 |
6.40 ± 0.10 |
97.15 ± 2.05 |
463.00 ± 54.30 |
25.40 ± 1.10 |
|
F12 |
Clear |
11.80 ± 1.20 |
6.00 ± 0.30 |
98.40 ± 3.30 |
970.00 ± 66.20 |
28.30 ± 3.70 |
Viscosity and rheological determination
The viscosity of all ISG formulations was around 21.7 ± 1.9 to 5040.4 ± 98.2 cP. F7 was selected for further evaluation among all formulations as it revealed appropriate physical appearance, gel capacity, pH, %drug content, and viscosity. The study used an oscillatory temperature sweep on optimal GCR-NS-loaded ISG mucoadhesive eye drops (F7) to determine its thermos-gelling behavior, represented in Figure 3. Storage modulus (G’) and loss modulus (G”) were used to measure the elasticity and viscosity of ISG, respectively. Gelation temperature was determined by the crossover point of G’ and G” and was found to be 35.98 ± 1.91°C.

Figure 3. Gelation temperature of optimal GCR-NS-loaded ISG mucoadhesive eye drops.
Mucoadhesive property
The possible synergistic mucoadhesive effect of the polymer combination was evaluated by measuring adhesive force, as represented in Table 6. The results revealed that the adhesive force of the P127 formulation increased when polymer concentration increased. Furthermore, the adhesive force of the P127 contained HA and HA-cat was significantly higher than that of the P127 formulation alone (1.1 ± 0.1 N) and GCR-NS (0.3 ± 0.1 N).
In vitro release study and kinetics
The drug release profiles of GCR suspension, GCR-NS, GCR-NS-loaded ISG formulation, and GCR-NS-loaded ISG mucoadhesive formulation are depicted in Figure 4. The percentage cumulative drug release from GCR suspension and GCR-NS reached nearly 100% within 6 hours. In contrast, the GCR-NS-loaded ISG formulation and GCR-NS-loaded ISG mucoadhesive formulation exhibited sustained drug release for over 24 hours, with approximately 70% of the drug released. Mathematical kinetic models were used to explain the behavior of drug release. The release kinetic behavior of GCR suspension and GCR-NS were First-order kinetic with R2 about 0.9942 and 0.9729, respectively. In contrast, the GCR-NS-loaded ISG formulation and GCR-NS-loaded ISG mucoadhesive formulation showed a Higuchi release kinetic with R2 about 0.9628 and 0.9745, respectively. The R2 of each kinetic model is shown in Table 7.
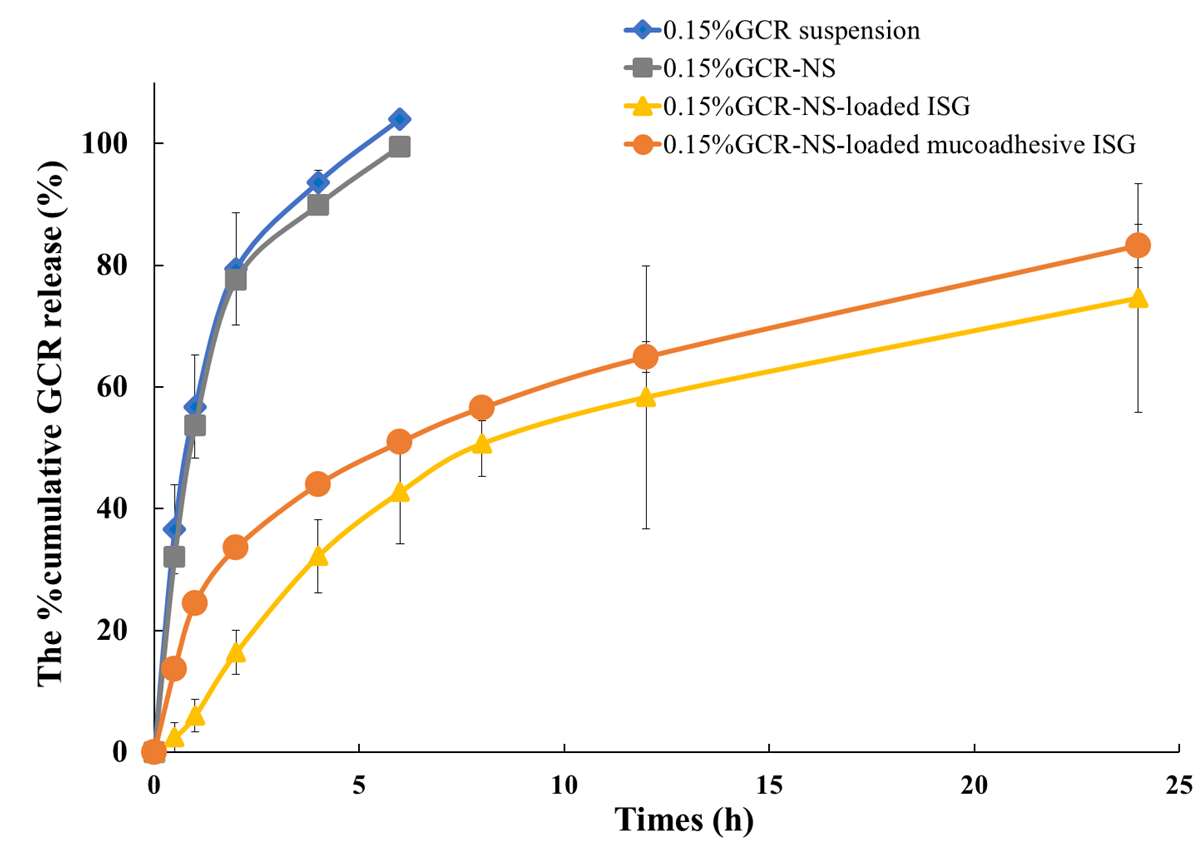 3
3
Figure 4. Drug release profiles of GCR suspension, GCR-NS, GCR-NS-loaded ISG formulation, and GCR-NS-loaded ISG mucoadhesive formulation
Table 7. The R2 of selecting the best-fit release kinetic models.
|
Formulations |
R2 value |
|||
|
Zero-order |
First-order |
Higuchi |
Korsemeyer-Peppas |
|
|
GCR suspension |
0.7558 |
0.9942* |
0.9481 |
0.3168 |
|
GCR-NS |
0.7795 |
0.9729* |
0.9555 |
0.3345 |
|
GCR-NS-loaded ISG |
0.8318 |
0.9489 |
0.9628* |
0.8135 |
|
GCR-NS-loaded mucoadhesive ISG |
0.7973 |
0.9638 |
0.9745* |
0.5090 |
The best-fit release kinetic model with the highest R2
In vitro corneal permeation study and drug remaining in porcine cornea
The optimized GCR-NS loaded mucoadhesive ISG formulation (F7) demonstrated significantly greater %GCR permeation than other formulations, as represented in Figure 5. The GCR from the 0.15% GCR-NS loaded mucoadhesive ISG (F7) permeated approximately 85.84 ± 16.68% over 24 h, while GCR permeated from 0.15% GCR suspension, 0.15% GCR-NS, and 0.15% GCR-NS-loaded ISG were 10.89 ± 2.83%, 35.15 ± 21.78%, and 61.28 ± 32.04%, respectively. Likewise, the flux of 0.15% GCR-NS loaded mucoadhesive ISG (30.65±7.11 µg/cm2/h) was significantly higher than other formulations. However, the percentage of GCR remaining in the corneal tissue over 24 h did not show a significant difference between the formulations
(18.50 ± 3.88% to 23.86 ± 3.91%).

Figure 5. %GCR permeation (A) and Flux of GCR suspension, GCR-NS, GCR-NS-loaded ISG formulation, and GCR-NS-loaded ISG mucoadhesive formulation(B). * Significantly higher than other formulations (P < 0.05).
DISCUSSION
It is well-established to obtain a pronounced effect against HSV eye infections, GCR must reach therapeutic concentrations in the cornea, which is the location of virus propagation (Ahmad et al., 2023). Therefore, penetration and retention of GCR in the cornea tissue is an important concern for the successful treatment of herpes keratitis. Ganciclovir (GCR) is categorized as a BSC class III drug, and topical eye drop GCR formulation had a low distribution coefficient and permeability (Parr et al., 2016). Therefore, to reach therapeutic concentrations in the cornea tissue, conventional management, such as oral medication for herpes keratitis, requires high-dose administration of 3 g/day for 7-14 days for herpetic keratitis treatment. In addition, it is associated with serious side effects such as bone marrow suppression and neutropenia (S. Akhter et al., 2012). Therefore, it has been suggested that GCR topical treatment has low efficacy due to low drug penetration to the target tissue. Therefore, administration of 0.15% GCR ophthalmic gel for one drop into the affected eye 5 times daily until healing of a corneal ulcer, followed by one drop three times daily for 7 days, was recommended resulting in low patient compliance. Our findings suggest that GCR-NS-loaded in situ gelling mucoadhesive eye drops (GCR-NS-loaded ISG) could be a useful delivery system that could enhance the permeation of topical viral infections by GCR.
Optimized GCR-NS formulation prepared using nanoprecipitation technique was selected based on CCD by evaluating their particle size, PDI, and zeta potential. The particle size was an important physicochemical parameter that could affect biopharmaceutical properties, such as solubility, dissolution, and drug permeation (Yadollahi et al., 2015). Response surface graphs (2D and 3D) were used to investigate the interactions and relationships of each parameter, as shown in Figure 1. The PVA had a positive effect on the particle size, while the SLS had a negative effect, with the antisolvent found to be insignificant. The results indicated that the particle size increased when the amount of PVA increased or SLS decreased. In addition, PVA has a greater influence than SLS, possibly due to the bridging effect obtained from higher amounts of stabilizer (Kobierski et al., 2009; Suriyaamporn et al., 2023).
PDI, which referred to the width of the particle size distribution, was one more important factor affecting nanosuspension’s homogeneity. The acceptable value for nanosuspension PDI is less than 0.5 (Wang et al., 2013; Celebi et al., 2019). The regression equation and response surface graphs showed that PVA positively affected PDI, while SLS and the antisolvent had negative effects. The finding suggested that PDI increased when increasing the amount of PVA or decreasing SLS and antisolvent (Liu et al., 2011; Li et al., 2021). Zeta potential affects GCR-NS stability. Stable NS should have zeta potential values between ±20 to ±40 mV (Oktay et al., 2018). The regression equation and response surface graphs of zeta potential showed that this model could not predict zeta potential due to low R2 and the observed inappropriate lack of fit value. Theoretically, the trend of zeta potential could be observed in correspondence with the charge of the stabilizer, PVA, and SLS, anionic stabilizers. Accordingly, the negative charge on the particle surface was increased with increasing amounts of PVA and SLS. Forming a negative ion barrier of NS could inhibit particle aggregation, particle growth, or Ostwald ripening. In this study, the negative charge of particles mostly resulted from PVA, which was used in higher amounts than SLS. According to the results, the optimal GCR-NS formulation with high desirability was the combination of 150.00 mg of PVA, 8.845 mg of SLS, and 11.37 mL of anti-solvent. As confirmed by TEM, GCR-NS formulation could reduce the particle size compared to coarse GCR powder. Moreover, stabilizers could help prevent particle aggregation, as stabilizer molecules were absorbed on the surface of GCR-NS particles and surrounding the drug particles (Yao et al., 2013).
All formulations of GCR-NS loaded ISG were rapidly gel-forming upon contact with simulated tear fluid at 37 °C. Moreover, the gelation temperature was determined by the crossover point of G' and G". It was found to be 35.98 ± 1.91°C, which is close to the human tear temperature of about 35.20 ± 0.45°C, making it suitable for clinical applications (Shah et al., 2021). The pH of formulations (in the range of 6.5-7.5) was ideal for practical use and was safe on the ocular tissue to ensure patient comfort (acceptable pH range 6.5-7.8). (Abelson et al., 1981; Che Arif et al., 2020; Mohamed-Ahmed et al., 2023). All formulations are clear, which can increase patient compliance. Moreover, high quantities of GCR were successfully loaded into the ISG, with no significant difference in each formulation. To achieve effective ophthalmic drug delivery, the ideal viscosity range is 30-300 mPas, as higher viscosity could reduce drug release and cause discomfort, while lower viscosity could lead to reduced corneal residence time (Che Arif et al., 2020; Ahmed et al., 2023). The results showed that increased concentrations of Pluronic® F127 (P127) polymer resulted in the increased viscosity of the ISG formulation. P127 is suitable for gel formation on the ocular surface as the spontaneous gelation temperature was around 36 °C. Moreover, when the temperature of the ISG formulations increased, the modulus values also increased, indicating a thermo-responsive sol-gel transition (Freitas et al., 2009; Fakhari et al., 2017).
Mucoadhesion was one of the crucial factors in extending the residence time of eye drops on the ocular surface, thereby reducing precorneal elimination. According to P127, it exhibited poor mucoadhesive properties (Ci et al., 2017); combining it with good mucoadhesive polymers could improve retention time. Incorporating HA or HA-cat into P127-based formulations was expected to enhance mucoadhesion; however, increasing the amount of mucoadhesive polymer led to an increase in the viscosity of the eye formulation, making it unsuitable for use on the eye (Permana et al., 2021; Conejo-Cuevas et al., 2022). Therefore, 0.15% GCR-NS loaded mucoadhesive ISG (F7), consisting of 10% P127 and 0.1% HA-cat, was selected for further evaluation as it revealed appropriate viscosity, mucoadhesion, and pH. The in vitro drug release study showed that the ISG formulation demonstrated sustained release over 24 h, following the Higuchi model release kinetics, which explains the drug release from a solid matrix system. In contrast, the GCR suspension and GCR-NS showed a burst release effect within 6 h, which can be explained by first-order kinetics (where the drug release rate depends on concentration). Thus, the ISG formulation could reduce the burst release effect of GCR-NS and sustained GCR release over 24 h (Suriyaamporn et al., 2023). In the in vitro corneal permeation study, the ISG formulation demonstrated enhanced permeation of GCR across the cornea tissue. Improving the permeation effect of ISG formulation could be due to the role of the non‐ionic surfactant, P127, to interact with the epithelial ocular membrane tissue (Grimaudo et al., 2018). Moreover, the mucoadhesive ISG formulation exhibited permeation significantly higher than the ISG formulation. This outcome could potentially be attributed to the synthesis of a new functional group (catechol) in HA-cat, which possesses a negative charge that can interact with ocular structures and enhance GCR permeation. (G. M. Soliman et al., 2014). The mucoadhesive ISG added into the formulation could be attributed to the nanoparticle accumulation in the conjunctival sac and the phase transition of in situ gel, which formed a depot resulting in the drug being slowly delivered to the precorneal area (Wen et al., 2018; Szalai et al., 2022). The therapeutic concentration required to treat herpetic keratitis, as indicated by the IC50, was approximately 24.3-691.0 ng/mL. A recent study on the pharmacokinetics of GCR in rabbit eyes revealed that 0.5% GCR eye drop was rapidly distributed into the cornea. One hour after administration, the mean GCR distribution level in the cornea was 28.0 ± 4.3 µg/g. This observation corresponds to an in vitro ocular permeation study. However, the GCR concentration was quickly decreased, eventually reaching 4.3 ± 2.1 µg/g in the cornea after four hours. These results were attributed to the tear turnover rate of about 0.5-2.2 mL/min. Therefore, mucoadhesive ISG can improve ex vivo transcorneal penetration compared to the GCR eye drop and ISG formulation. Our study indicated that the ganciclovir concentration was higher than the IC50 level, providing additional pharmacokinetic data supporting the clinical observation. Consequently, these GCR-NS-loaded ISG formulations were in range and may have the potential to treat herpetic keratitis (Sohail Akhter et al., 2013; Chou et al., 2014; Okumura et al., 2019).
CONCLUSION
The studies presented here demonstrate the potential of GCR-NS-loaded mucoadhesive ISG to improve ocular drug permeation. This work, therefore, reflects an advantage of NS combined with mucoadhesive ISG for enhancing the local treatment of a drug with poor permeability, a typical limitation of topical drugs. Compared with other formulations, the results suggested that the optimized GCR-NS-loaded mucoadhesive ISG had better viscosity, mucoadhesion, and gelling capacity at physiological conditions. Moreover, the GCR-NS-loaded mucoadhesive ISG exhibited prolonged and high ocular GCR permeation within 24 h compared to eye drop suspension, reducing the dosing frequency. The research demonstrated that an optimized GCR-NS-loaded mucoadhesive ISG could be a promising ocular delivery system for effective local delivery of GCR. The value of expansion in clinical use is likely to be considerable; evaluation of antiviral activity, irritation tests, and undergoing in vivo studies is now important to ensure that they do not act as a barrier to clinical use and, therefore, successful commercialization.
ACKNOWLEDGMENTS
This research was funded by the National Research Council of Thailand (NRCT): N42A650551 and the Research and Creative Fund, Faculty of Pharmacy, Silpakorn University.
AUTHOR CONTRIBUTIONS
Phuvamin Suriyaamporn: methodology, software, validation, formal analysis, investigation, data curation, and writing-original draft preparation. Boonnada Pamornpathomkul: writing-review and editing. Theerasak Rojanarata: resources, visualization. Praneet Opanasopit: conceptualization, project administration, and funding acquisition. Prasopchai Patrojanasophon: design of methodology. Tanasait Ngawhirunpat: visualization, supervision, and project administration. All authors have read and agreed to the published version of the manuscript.
CONFLICT OF INTEREST
The authors declare that they hold no competing interests.
REFERENCES
Abbas, M. N., Khan, S. A., Sadozai, S. K., Khalil, I. A., Anter, A., Fouly, M. E., et al. 2022. Nanoparticles loaded thermoresponsive in situ gel for ocular antibiotic delivery against bacterial keratitis. Polymers. 14(6): 1135.
Abelson, M. B., Udell, I. J., and Weston, J. H. 1981. Normal human tear pH by direct measurement. Archives of Ophthalmology. 99(2): 301-301.
Ahmad, B., and Patel, B. C. 2023. Herpes simplex keratitis. In StatPearls. Treasure Island (FL) ineligible companies. Disclosure: Bhupendra Patel declares no relevant financial relationships with ineligible companies. StatPearls Publishing LLC.
Ahmed, S., Amin, M. M., and Sayed, S. 2023. Ocular drug delivery: A comprehensive review. American Association of Pharmaceutical Scientists. 24(2): 66.
Akhter, S., Kushwaha, S., Warsi, M. H., Anwar, M., Ahmad, M. Z., Ahmad, I., et al. 2012. Development and evaluation of nanosized niosomal dispersion for oral delivery of ganciclovir. Drug Development and Industrial Pharmacy. 38(1): 84-92.
Akhter, S., Ramazani, F., Ahmad, M. Z., Ahmad, F. J., Rahman, Z., Bhatnagar, A., et al. 2013. Ocular pharmacoscintigraphic and aqueous humoral drug availability of ganciclovir-loaded mucoadhesive nanoparticles in rabbits. European Journal of Nanomedicine. 5(3): 159-167.
Al-Kinani, A. A., Zidan, G., Elsaid, N., Seyfoddin, A., Alani, A. W. G., and Alany, R. G. 2018. Ophthalmic gels: Past, present and future. Advanced Drug Delivery Reviews. 126: 113-126.
Amorós, G. L., Nardi, R. A., Verdugo, G. C., Arroyo, G. C. M., García, M. E., Pérez, L. P., et al. 2022. Development of a standardized method for measuring bioadhesion and mucoadhesion that is applicable to various pharmaceutical dosage forms. Pharmaceutics. 14(10): 1995.
Arvidson, S. A., Lott, J. R., McAllister, J. W., Zhang, J., Bates, F. S., Lodge, T. P., et al. 2013. Interplay of phase separation and thermoreversible gelation in aqueous methylcellulose solutions. Macromolecules. 46(1): 300-309.
Baranowski, P., Karolewicz, B., Gajda, M., and Pluta, J. 2014. Ophthalmic drug dosage forms: Characterisation and research methods. The Scientific World Journal. 2014: 861904.
Beard, M., Cobb, L., Grant, C., Varadarajan, A., Henry, T., Swanson, E., et al. 2020. Autoclaving of poloxamer 407 hydrogel and its use as a drug delivery vehicle. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 109(3): 338-347.
Cao, F., Zhang, X., and Ping, Q. 2010. New method for ophthalmic delivery of azithromycin by poloxamer/carbopol-based in situ gelling system. Drug Delivery. 17(7): 500-507.
Celebi, N., and Gülbağ, S. 2019. Optimization and evaluation of cyclosporine A nanosuspension stabilized by combination stabilizers using high pressure homogenization method. Sanat Tasarim Dergisi. 23: 1009-1021.
Che Arif, F., Hilmi, M. R., Kamal, K., and Ithnin, M. 2020. Evaluation of 18 artificial tears based on viscosity and pH. Malaysian Journal of Ophthalmology. 2: 96-111.
Chou, T. Y., and Hong, B. Y. 2014. Ganciclovir ophthalmic gel 0.15% for the treatment of acute herpetic keratitis: Background, effectiveness, tolerability, safety, and future applications. Therapeutics and Clinical Risk Management. 10: 665-681.
Ci, L., Huang, Z., Liu, Y., Liu, Z., Wei, G., and Lu, W. 2017. Amino-functionalized poloxamer 407 with both mucoadhesive and thermosensitive properties: Preparation, characterization and application in a vaginal drug delivery system. Acta Pharmaceutica Sinica B. 7(5): 593-602.
Conejo-Cuevas, G., Ruiz-Rubio, L., Sáez-Martínez, V., Pérez-González, R., Gartziandia, O., Huguet-Casquero, A., et al. 2022. Spontaneous gelation of adhesive catechol modified hyaluronic acid and chitosan. Polymers. 14(6): 1209.
Cook, M. T., Abou-Shamat, M. A., Stair, J. L., and Calvo-Castro, J. 2022. Raman spectroscopy coupled to computational approaches towards understanding self-assembly in thermoreversible poloxamer gels. Journal of Molecular Liquids. 351: 118660.
Elmowafy, E., Cespi, M., Bonacucina, G., and Soliman, M. E. 2019. In situ composite ion-triggered gellan gum gel incorporating amino methacrylate copolymer microparticles: A therapeutic modality for buccal applicability. Pharmaceutical Development and Technology. 24(10): 1258-1271.
Fakhari, A., Corcoran, M., and Schwarz, A. 2017. Thermogelling properties of purified poloxamer 407. Heliyon. 3(8): e00390.
Fangueiro, J., Veiga, F., M Silva, A., and B Souto, E. 2016. Ocular drug delivery-new strategies for targeting anterior and posterior segments of the eye. Current Pharmaceutical Design. 22(9): 1135-1146.
Farooq, A. V., and Shukla, D. 2012. Herpes simplex epithelial and stromal keratitis: An epidemiologic update. Survey of Ophthalmology. 57(5): 448-462.
Freitas, M., Farah, M., Bretas, R., E, R.-J., and Marchetti, J. 2009. Rheological characterization of Poloxamer 407 nimesulide gels. Revista de Ciências Farmacêuticas Básica e Aplicada. 27(2).
Fukuda, I. M., Pinto, C. F. F., Moreira, C. d. S., Saviano, A. M., and Lourenço, F. R. 2018. Design of Experiments (DoE) applied to Pharmaceutical and Analytical Quality by Design (QbD). Brazilian Journal of Pharmaceutical Sciences. 54.
Galante, R., Pinto, T. J. A., Colaço, R., and Serro, A. P. 2018. Sterilization of hydrogels for biomedical applications: A review. Journal of Biomedical Materials Research - Part B Applied Biomaterials. 106(6): 2472-2492.
Gote, V., Sikder, S., Sicotte, J., and Pal, D. 2019. Ocular drug delivery: Present innovations and future challenges. Journal of Pharmacology and Experimental Therapeutics. 370(3): 602.
Grimaudo, M. A., Pescina, S., Padula, C., Santi, P., Concheiro, A., Alvarez-Lorenzo, C., et al. 2018. Poloxamer 407/TPGS mixed micelles as promising carriers for cyclosporine ocular delivery. Molecular Pharmaceutics. 15(2): 571-584.
Gupta, H., Aqil, M., Khar, R. K., Ali, A., Bhatnagar, A., and Mittal, G. 2013. Nanoparticles laden in situ gel for sustained ocular drug delivery. Journal of Pharmacy and Bioallied Sciences. 5(2): 162-165.
Gupta, H., Jain, S., Mathur, R., Mishra, P., Mishra, A. K., and Velpandian, T. 2007. Sustained ocular drug delivery from a temperature and pH triggered novel in situ gel system. Drug Delivery. 14(8): 507-515.
Haridas, N., and Rosemary, M. J. 2019. Effect of steam sterilization and biocompatibility studies of hyaluronic acid hydrogel for viscosupplementation. Polymer Degradation and Stability. 163: 220-227.
Hoh, H. B., Hurley, C., Claoue, C., Viswalingham, M., Easty, D. L., Goldschmidt, P., et al. 1996. Randomised trial of ganciclovir and acyclovir in the treatment of herpes simplex dendritic keratitis: A multicentre study. British Journal of Ophthalmology. 80(2): 140-143.
Jakubowska, E., Bielejewski, M., Milanowski, B., and Lulek, J. 2022. Freeze-drying of drug nanosuspension– study of formulation and processing factors for the optimization and characterization of redispersible cilostazol nanocrystals. Journal of Drug Delivery Science and Technology. 74: 103528.
Kassem, M. A., Abdel Rahman, A. A., Ghorab, M. M., Ahmed, M. B., and Khalil, R. M. 2007. Nanosuspension as an ophthalmic delivery system for certain glucocorticoid drugs. International Journal of Pharmaceutics. 340(1-2): 126-133.
Khare, P., Chogale, M. M., Kakade, P., and Patravale, V. B. 2022. Gellan gum–based in situ gelling ophthalmic nanosuspension of Posaconazole. Drug Delivery and Translational Research. 12(12): 2920-2935.
Kim, J., Lee, C., and Ryu, J. H. 2021. Adhesive catechol-conjugated hyaluronic acid for biomedical applications: A mini review. Applied Sciences. 11(1).
Kobierski, S., Ofori-Kwakye, K., Müller, R. H., and Keck, C. M. 2009. Resveratrol nanosuspensions for dermal application--production, characterization, and physical stability. Pharmazie. 64(11): 741-747.
Kuk, D. H., Ha, E. S., Ha, D. H., Sim, W. Y., Lee, S. K., Jeong, J. S., et al. 2019. Development of a resveratrol nanosuspension using the antisolvent precipitation method without solvent removal, based on a quality by design (QbD) approach. Pharmaceutics. 11(12): 1.
Li, J., Wang, Z., Zhang, H., Gao, J., and Zheng, A. 2021. Progress in the development of stabilization strategies for nanocrystal preparations. Drug Deliverv. 28(1): 19-36.
Liesegang, T. J. 2001. Herpes simplex virus epidemiology and ocular importance. Cornea. 20(1): 1-13.
Lin, T., Gong, L., Sun, X. H., Zhao, N. Q., Chen, W., Yuan, H. P., et al. 2013. Effectiveness and safety of 0.15% ganciclovir in situ ophthalmic gel for herpes simplex keratitis - a multicenter, randomized, investigator-masked, parallel group study in Chinese patients. Drug Design, Development and Therapy. 7: 361-368.
Liu, P., Rong, X., Laru, J., van Veen, B., Kiesvaara, J., Hirvonen, J., et al. 2011. Nanosuspensions of poorly soluble drugs: Preparation and development by wet milling. International Journal of Pharmaceutics. 411(1): 215-222.
Majeed, A., and Khan, N. 2019. Ocular in situ gel: An overview. Journal of Drug Delivery and Therapeutics. 9: 337-347.
Merodio, M., Campanero, M. A., Mirshahi, T., Mirshahi, M., and Irache, J. M. 2000. Development of a sensitive method for the determination of ganciclovir by reversed-phase high-performance liquid chromatography. Journal of Chromatography A. 870(1): 159-167.
Mohamed-Ahmed, A. H. A., and Kuguminkiriza, D. 2023. Local production of eye drops in the hospital or pharmacy setting: considerations and safety tips. Community Eye Health. 36(118): 17-18.
Moore, D. S., Notz, W., and Fligner, M. A. 2013. The Basic Practice of Statistics: W.H. Freeman and Company.
Naito, T., Shiota, H., and Mimura, Y. 1987. Side effects in the treatment of herpetic keratitis. Current Eye Research. 6(1): 237-239.
Oktay, A. N., Karakucuk, A., Ilbasmis-Tamer, S., and Celebi, N. 2018. Dermal flurbiprofen nanosuspensions: Optimization with design of experiment approach and in vitro evaluation. European Journal of Pharmaceutical Sciences. 122: 254-263.
Okumura, N., Tanaka, T., Fukui, Y., and Koizumi, N. 2019. Stability, safety, and pharmacokinetics of ganciclovir eye drops prepared from ganciclovir for intravenous infusion. Japanese Journal of Ophthalmology. 63(3): 289-296.
Pandey, M., Choudhury, H., Abdul-Aziz, A., Bhattamisra, S. K., Gorain, B., Su, J. S. T., et al. 2020. Advancement on sustained antiviral ocular drug delivery for herpes simplex virus keratitis: Recent update on potential investigation. Pharmaceutics. 13(1): 1.
Parr, A., Hidalgo, I. J., Bode, C., Brown, W., Yazdanian, M., Gonzalez, M. A., et al. 2016. The effect of excipients on the permeability of BCS class III compounds and implications for biowaivers. Pharmaceutical Research. 33(1): 167-176.
Permana, A. D., Utami, R. N., Layadi, P., Himawan, A., Juniarti, N., Anjani, Q. K., et al. 2021. Thermosensitive and mucoadhesive in situ ocular gel for effective local delivery and antifungal activity of itraconazole nanocrystal in the treatment of fungal keratitis. International Journal of Pharmaceutics. 602: 120623.
Pınar, S. G., Oktay, A. N., Karaküçük, A. E., and Çelebi, N. 2023. Formulation strategies of nanosuspensions for various administration routes. Pharmaceutics. 15(5): 1520.
Pornpitchanarong, C., Rojanarata, T., Opanasopit, P., Ngawhirunpat, T., and Patrojanasophon, P. 2020. Catechol-modified chitosan/hyaluronic acid nanoparticles as a new avenue for local delivery of doxorubicin to oral cancer cells. Colloids and Surfaces B: Biointerfaces. 196: 111279.
Shah, A. M., and Galor, A. 2021. Impact of ocular surface temperature on tear characteristics: Current insights. Clinical Optometry. 13: 51-62.
Soliman, G. M., Zhang, Y. L., Merle, G., Cerruti, M., and Barralet, J. 2014. Hydrocaffeic acid–chitosan nanoparticles with enhanced stability, mucoadhesion and permeation properties. European Journal of Pharmaceutics and Biopharmaceutics. 88(3): 1026-1037.
Soliman, K. A., Ullah, K., Shah, A., Jones, D. S., and Singh, T. R. R. 2019. Poloxamer-based in situ gelling thermoresponsive systems for ocular drug delivery applications. Drug Discovery Today. 24(8): 1575-1586.
Soltani, S., Zakeri-Milani, P., Barzegar-Jalali, M., and Jelvehgari, M. 2016. Comparison of different nanosuspensions as potential ophthalmic delivery systems for ketotifen fumarate. Advanced Pharmaceutical Bulletin. 6(3): 345-352.
Suriyaamporn, P., Opanasopit, P., Ngawhirunpat, T., and Rangsimawong, W. 2021. Computer-aided rational design for optimally Gantrez® S-97 and hyaluronic acid-based dissolving microneedles as a potential ocular delivery system. Journal of Drug Delivery Science and Technology. 61: 102319.
Suriyaamporn, P., Opanasopit, P., Rangsimawong, W., and Ngawhirunpat, T. 2022. Optimal design of novel microemulsions-based two-layered dissolving microneedles for delivering fluconazole in treatment of fungal eye infection. Pharmaceutics. 14(3): 472.
Suriyaamporn, P., Sahatsapan, N., Patrojanasophon, P., Opanasopit, P., Kumpugdee-Vollrath, M., and Ngawhirunpat, T. 2023. Optimization of in situ gel-forming chlorhexidine-encapsulated polymeric nanoparticles using design of experiment for periodontitis. AAPS PharmSciTech. 24(6): 161.
Szalai, B., Jójárt-Laczkovich, O., Kovács, A., Berkó, S., Balogh, G. T., Katona, G., et al. 2022. Design and optimization of in situ gelling mucoadhesive eye drops containing dexamethasone. Gels. 8(9): 561.
Tabbara, K. F., and Al Balushi, N. 2010. Topical ganciclovir in the treatment of acute herpetic keratitis. Clinical Ophthalmology. 4: 905-912.
Wang, N., Qi, F., He, X., Shi, H., Anderson, D. W., Li, H., et al. 2021. Preparation and pharmacokinetic characterization of an anti-virulence compound nanosuspensions. Pharmaceutics. 13(10): 1586.
Wang, Y., Zheng, Y., Zhang, L., Wang, Q., and Zhang, D. 2013. Stability of nanosuspensions in drug delivery. Journal of Controlled Release. 172(3): 1126-1141.
Wen, Y., Ban, J., Mo, Z., Zhang, Y., An, P., Liu, L., et al. 2018. A potential nanoparticle-loaded in situ gel for enhanced and sustained ophthalmic delivery of dexamethasone. Nanotechnology. 29(42): 425101.
White, M., Chodosh, J., Street, C., Feder, R. S., Pavan-Langston, D., LiesegaT. J., et al. 2014. Herpes simplex virus keratitis: A treatment guideline. American Academy of Ophthalmology.
Yadollahi, R., Vasilev, K., and Simovic, S. 2015. Nanosuspension technologies for delivery of poorly soluble drugs. Journal of Nanomaterials. 2015: 216375.
Yao, Q., Tao, X., Tian, B., Tang, Y., Shao, Y., Kou, L., et al. 2013. Improved oral bioavailability of core-shell structured beads by redispersion of the shell-forming nanoparticles: Preparation, characterization and in vivo studies. Colloids and surfaces. B, Biointerfaces. 113C: 92-100.
Zhang, X., Wei, D., Xu, Y., and Zhu, Q. 2021. Hyaluronic acid in ocular drug delivery. Carbohydrate Polymers. 264: 118006.
Zielińska, A., Soles, B. B., Lopes, A. R., Vaz, B. F., Rodrigues, C. M., Alves, T. F. R., et al. 2020. Nanopharmaceuticals for eye administration: sterilization, depyrogenation and clinical applications. Biology. 9(10): 336.
OPEN access freely available online
Natural and Life Sciences Communications
Chiang Mai University, Thailand. https://cmuj.cmu.ac.th
Phuvamin Suriyaamporn, Boonnada Pamornpathomkul, Theerasak Rojanarata, Prasopchai Patrojanasophon, Praneet Opanasopit, and Tanasait Ngawhirunpat*
Pharmaceutical Development of Green Innovations Group (PDGIG), Faculty of Pharmacy, Silpakorn University, Nakhon Pathom 73000, Thailand.
Corresponding author: Tanasait Ngawhirunpat, E-mail: ngawhirunpat_t@su.ac.th
Total Article Views
Editor: Wipawadee Yooin,
Chiang Mai University, Thailand
Article history:
Received: April 13, 2023;
Revised: September 19, 2023;
Accepted: October 11, 2023;
Online First: October 31, 2023

