
Bloom Performances during Rainy Season of Off-season Longan (Dimocarpus longan Lour. ‘Phuang Thong’) Grown at Samut Sakhon Province, Thailand
Phyu Phyu Aung, Nareenart Traisuwan, Watcharra Chintakovid, Tatpong Tulyananda, and Aussanee Pichakum*Published Date : October 30, 2023
DOI : https://doi.org/10.12982/NLSC.2023.069
Journal Issues : Number 4, October-December 2023
Abstract Rainy season is an obstruction for efficient off-season longan production in Thailand. In this experiment, the bloom performances of off-season 'Phuang Thong' longan as affected by microclimate in a private longan orchard at Ban Phaeo district, Samut Sakhon province was evaluated during the rainy season of 2020. This study focused on the reproductive development at four different times (plots) of potassium chlorate application: May, June, July, and August. Air temperature, relative humidity and light intensity at the canopy level were recorded. Rainfall data was obtained from the Meteorological Department of Thailand. The results showed that inflorescence length, female flower number, and sex ratio of female to male of the May plot were the highest compared to other plots, and it was associated with early rainfall occurrence during flower development and blooming time. The August plot had a low growing degree day and a short flowering period, which coincided with frequent rainfall during the flower development period. However, the pollen viability and female flower characters were unaffected by environment. In conclusion, flower induction in the early rainy season, especially in May, was an effective time for the production of off-season ‘Phuang Thong’ longan in Ban Phaeo district, Samut Sakhon province, Thailand.
Keywords: Floral biology, Flower, Pollen, Microclimate, Heat unit
Funding: This research was funded by the Thailand Science Research and Innovation Grant (grant number RDG6120031), The MUSC scholarship for Human Resource Development in Science and Technology in Honor of the Late King Rama IX of Thailand, and Mahidol University.
Citation: Aung, P.P., Traisuwan,N., Chintakovid, W., Tulyananda, T. and Pichakum, A. 2023. Bloom performances during rainy season of off-season longan (Dimocarpus longan Lour. ‘Phuang Thong’) grown at Samut Sakhon province, Thailand. Natural and Life Sciences Communications. 22(4): e2023069.
INTRODUCTION
In Thailand, longan (Dimocarpus longan Lour.) is an economically important fruit. Its irregular flowering is a major problem worldwide. The whole flowering process may take 6-9 months, depending on cultivar and environmental conditions (Thunyarpar, 1998). To increase profitability, most Thai longan growers now prefer cultivating off-season fruits by using a potassium chlorate treatment, which is quite efficient for flower induction throughout the year (Manochai et al., 2005). Well-developed techniques for off-season longan are regularly practiced (Subhadrabandhu and Yapwattanaphun, 2000). Jaroenkit and Manochai (2020) reported a significant increase in both in-season and off-season productivity in Thailand, with off-season longan accounting for about 50% of total production. The key factors for successful off-season longan production are technology, environment, and the farmer’s experience in cultivating longan, as well as strategic planning for high profit production timing.
In the fruit industry, it is important to control the flowering process in order to produce sufficient fruit as scheduled. Normally, longan floral induction occurs in winter, indicating that low temperature is a key factor. However, it is known that stresses such as chilling, water stress, or oxidative stress can induce flower in Sapindaceae such as litchi and longan. The roles of reactive oxygen species, nitric oxide, and chlorate-induced stress signals in Sapindaceae flowering is well understood (Zhou et al., 2014). As the application of low-concentration potassium chlorate is successful in the floral induction of many longan cultivars (Manochai et al., 2005; Zhou et al., 2014) within 21-28 days after treatment, this chemical plays a crucial role in flowering (Subhadrabandhu and Yapwattanaphun, 2000).
October to February is generally the most suitable time for potassium chlorate application in Thailand. During this period with low nighttime temperatures, the plant is in a dormant stage, which is when it is most responsive to chemical treatment (Subhadrabandhu and Yapwattanaphun, 2000). Season seems to have a decisive influence on flower induction effectiveness. A study on ‘E-Daw’ longan revealed that soil drench application had the highest success during the cool-dry season in northern Thailand and the least success during the rainy season (Manochai et al., 2005). From May to September, longan trees were not responsive to potassium chlorate treatment, with less than 50% flowering rate (Subhadrabandhu and Yapwattanaphun, 2000). It has been shown that rainfall just before the floral induction stage can reduce yield. Relative humidity between 64 - 68% can increase longan productivity, while higher humidity reduces the yield (Sritontip et al., 2014). A comparative study of potassium chlorate application revealed that rainy season (August) application resulted in a lower flowering percentage than summer (April) and winter (December) application (Manochai et al., 2010). The success of potassium chlorate flower induction depends on canopy size, method of application, and stage of leaf development. Beside these physical factors, suitable weather conditions should also be considered (Subhadrabandhu and Yapwattanaphun, 2000).
Longan growers in Samut Sakhon province, in the central region of Thailand, prefer off-season crops due to the high selling price, however, it also requires intensive orchard management. Because of high demand in late December and early February, inflorescence development occurring during the rainy season is faced with seasonal “hot wind” (Pichakum et al., 2020). However, the effects of microclimate on longan reproductive development in central Thailand are still unclear. This research aims to observe the bloom performances of off-season longan induced during the rainy season, as well as the microclimate conditions experienced in the field.
MATERIALS AND METHODS
Materials
Research was conducted during the rainy season of 2020 in a private orchard in Ban Phaeo district, Samut Sakhon province, Thailand (E13.592768, N100.069948). The 22-year old longan (Dimocarpus longan Lour. ‘Phuang Thong’) trees were grown in rows spaced between waterways (6x6 m spacing). Off-season production is usually induced by potassium chlorate during the rainy season to accommodate high winter demands. Table 1 presents the sequence of cultivation for off-season longan production. Soil drench with potassium chlorate at 26 g.m-2 of canopy was applied to 4 plots, each in a different month: May, June, July, and August (Table 1 and Figure 1). In 2020, the rainy season started 18 May (the 138th Julian date) and ended 22 October
(the 295th Julian date) (Meteorological Department of Thailand, 18 May 2020; 22 October 2020). The orchard was managed with usual practices comprising annual pruning, fertilizer application and crop protection.
Table 1. Off-season cultivation program of longan (Dimocarpus longan Lour. ‘Phuang Thong’) at Ban Phaeo district, Samut Sakhon province, Thailand in 2020.
|
Cultivation practice |
Plot name |
|||
|
May |
June |
July |
August |
|
|
Pruning time |
Early Feb. 2020 |
Early Mar. 2020 |
Early Apr. 2020 |
Early May 2020 |
|
Potassium chlorate application (Julian date) |
20 May 2020 (140th) |
19 Jun. 2020 (170th) |
16 Jul. 2020 (197th) |
13 Aug. 2020 (225th) |
|
Harvest time |
Late Dec. 2020 |
Late Jan. 2021 |
Late Feb. 2021 |
Mid. Mar. 2021 |
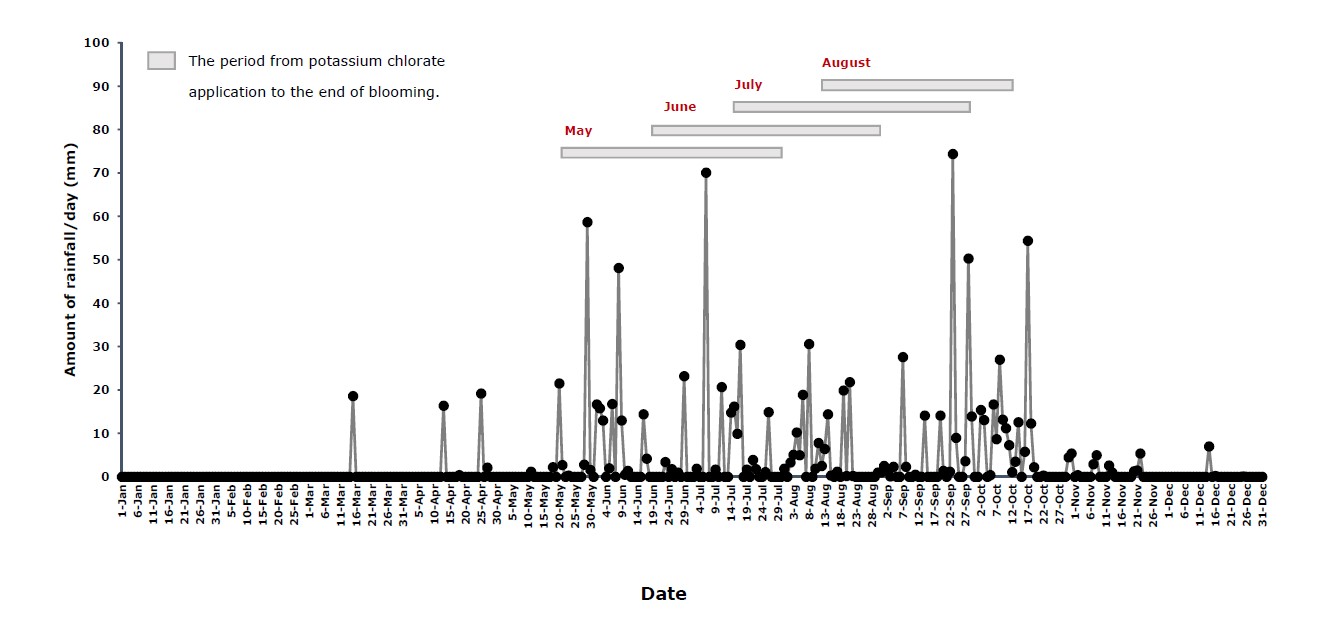
Figure 1. Daily rainfall (mm) at Ban Phaeo district, Samut Sakhon province, Thailand in 2020.
Source: Meteorological Department of Thailand.
Flowering performance
Ten uniform inflorescences from ten selected trees per plot time were randomly observed. Inflorescence length (cm) was measured every four days by tape measure, starting from inflorescence bud swelling (BBCH code 511) (Figure 2A) according to Pham et al. (2015b), until the end of panicle development (BBCH code 519) as the final inflorescence length (Figure 2B). Inflorescence performance was assessed by counting the total number of blooms in daily cut inflorescences with opened flowers, as well as identifying flower morphology/type in order to calculate the sex ratio.
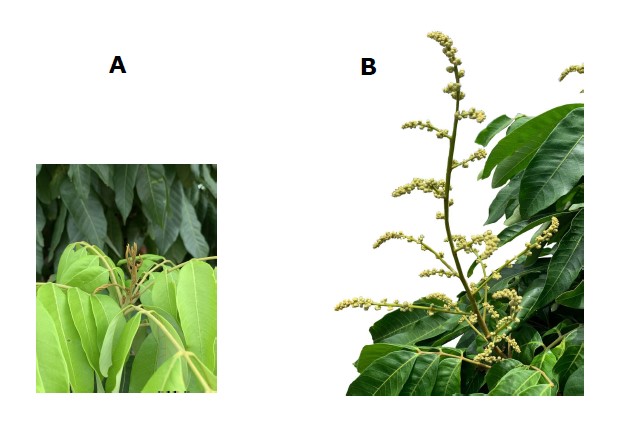
Figure 2. Inflorescence of off-season longan (Dimocarpus longan Lour. ‘Phuang Thong’) at Ban Phaeo district, Samut Sakhon province, Thailand in 2020. Photos of an inflorescence at BBCH code 511 (A) and an inflorescence at BBCH code 519 (B).
In each plot time, fifteen fully mature female flowers at the start of anthesis from ten uniform inflorescences were randomly collected in the morning for three days. One hundred fifty female flowers were pooled as representative samples of each study plot, and fixed in a mixture of 40% formalin, glacial acetic acid, and 70% ethanol (5:5:90) (Nacif et al., 2001), before being photographed under a light microscope according to Schneider et al. (2012). Digital images were analyzed using ImageJ software (v.1.8r, NIH, USA) to evaluate floral structure. Flower size was averaged using 5 measurements of the floral size (Figure 3A). Stigma width was measured as distance across the midpoint of the stigma (Figure 3B). Style length was measured from the tip to the base (Figure 3C). Ovary size was determined from the average of 2 perpendicular lines transecting the ovary (Figure 3D), and ovule size was measured in a similar manner (Figure 3E). Anther and petal number were counted.
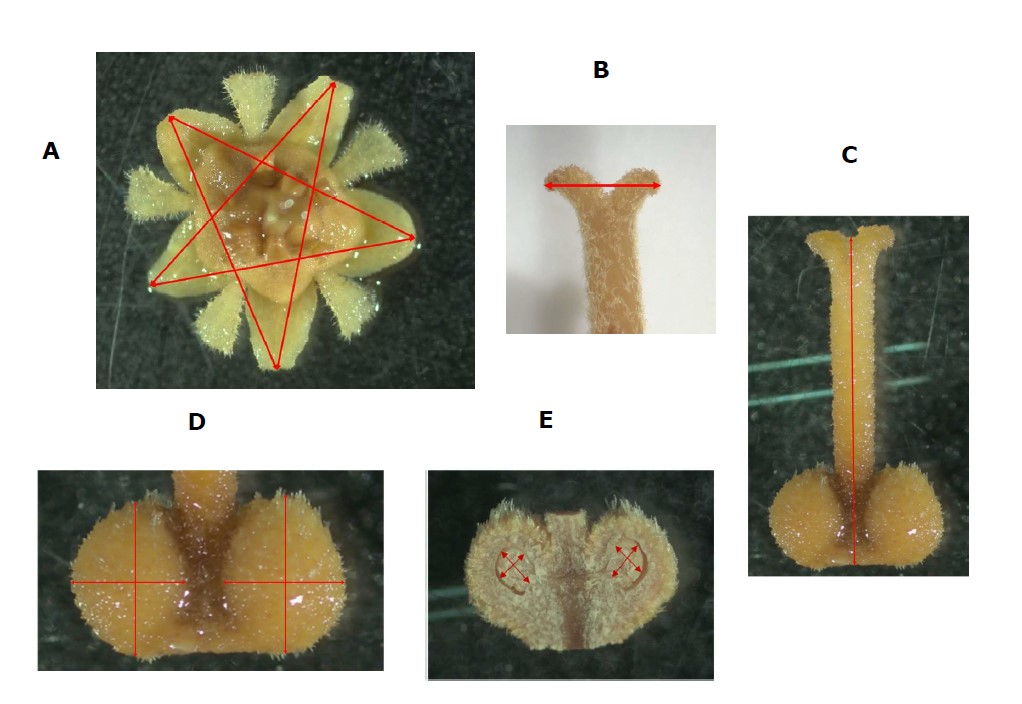
Figure 3. Evaluation of floral structures in off-season longan (Dimocarpus longan Lour. ‘Phuang Thong’) at Ban Phaeo district, Samut Sakhon province, Thailand in 2020. Red lines indicate measurements made for flower size (A); stigma width (B); style length (C); ovary size (D); ovule size (E).
Three hundred male flowers were randomly collected in the morning from ten uniform inflorescences prior to anther dehiscence (1 day before anthesis) for pollen germination and viability evaluation under a light microscope. Fresh anthers were lightly heated under a desk lamp overnight. A representative sample of pollen grains was prepared from a dehisced anther, placed in a drop of 2% acetocarmine on a glass slide, and covered with a coverslip. The numbers of stained and unstained pollen grains were counted to calculate the percentage of viable pollen. The rest of the pollen grains were cultured for 4 hours in a germination media modified from Thu et al. (2017) and Gupta et al. (2017) with a 15% sucrose concentration at room temperature. The numbers of germinated and non-germinated pollen tubes were used to determine the pollen germination rate.
Microclimate data
A weather micro-station with sensors and a data logger (Hobo, Onset Corp., USA) was installed at longan tree canopy level. The air temperature (°C), relative humidity (%), and light intensity (W/m2) were measured every 10 minutes starting from potassium chlorate application until the end of bloom. Collected data was analyzed using the Hoboware pro software (version 3.7.12, Onset Computer Corporation, USA). Rainfall data (mm) was obtained from Meteorological Department of Thailand. Days with more than 10 mm rainfall were considered rainy days. A heat sum unit or the growing degree day (GDD) (℃) of each plot during the floral development period was calculated following Jaroenkit et al. (2014).
Statistical analysis
Floral phenological data was statistically analyzed by one-way analysis of variance (ANOVA) and Tukey’s test to determine significant differences between plots. Air temperature, relative humidity and light intensity data were calculated separately among plots. Calculated temperature was analyzed in terms of the heat sum unit or GDD. Daily relative humidity was analyzed using minimum and maximum values. Daily light intensity was summed to represent the total amount of light. All statistical analyses were done using the IBM SPSS statistics program (Version 25.0).
RESULTS AND DISCUSSION
Inflorescence growth
Longan inflorescences are a type of compound dichasia with a high number of unisexual blooms, and the sex ratio varies depending on cultivar and environment (Subhadrabandhu and Stern, 2005). For off-season longan under field conditions at Ban Phaeo district, Samut Sakhon province, central Thailand with different potassium chlorate application times (plots), flowering occurred in order of chemical application, with flowers appearing in July, August, September and October, respectively (Figure 1). The final inflorescence length of the May plot was significantly longer (41.7 ± 1.0 cm) than the other plots; the inflorescence lengths of the June, July, and August plots were 31.1 ± 1.5, 30.0 ± 1.0, and 29.6 ± 0.5 cm, respectively (Table 2). The weather conditions of the May plot exhibited a binomial pattern of rainfall, with the 1st peak of 58.7 mm occurring during the early stage of inflorescence growth or the 10th day after potassium chlorate application (29 May 2020) (Figure 1), and this plot also had a high number of rainy days (16 days) and a high total amount of rainfall (Table 3). Moreover, the heat sum unit or the growing degree day (GDD) of the May plot was the high level of 1,338.6℃ (Table 3). Thus, the suitable weather conditions of warm temperature combined with high rainfall during the early stages of flowering supported off-season longan inflorescence development. This result corresponds with those of Sukhvibul et al. (2000), who reported that higher temperature generally increased inflorescence size while there was an inverse effect on inflorescence flower number in mango.
Table 2. Inflorescence length of off-season longan (Dimocarpus longan Lour. ‘Phuang Thong’) that developed at different times (May, June, July, and August) at Ban Phaeo district, Samut Sakhon province, Thailand in 2020.
|
Plot name |
Inflorescence length (cm) |
|
May |
41.7 ± 1.0 a |
|
June |
31.1 ± 1.5 b |
|
July |
30.0 ± 1.0 b |
|
August |
29.6 ± 0.5 b |
|
Significance |
* |
Table 3. Microclimate information collected starting from the time of potassium chlorate application to the end of blooming in off-season longan (Dimocarpus longan Lour. ‘Phuang Thong’) at Ban Phaeo district, Samut Sakhon province, Thailand in 2020. Potassium chlorate was applied to each of the four plots at different times (May, June, July, and August), with flowering occurring in July, August, September, and October.
|
Microclimate data |
Plot name |
|||
|
May |
June |
July |
August |
|
|
Rainy days (days) |
16 |
14 |
13 |
14 |
|
Total rainfall (mm) |
452.0 |
371.1 |
436.0 |
399.2 |
|
Growing degree day (℃) (from potassium chlorate application to the end of bloom) |
1,338.6 |
1,353.4 |
1,429.7 |
1,096.4 |
|
Relative humidity (%) (minimum) |
57.3 |
57.4 |
59.3 |
61.4 |
|
Relative humidity (%) (average) |
80.6 |
80.2 |
81.4 |
82.7 |
|
Total light intensity (W/m2) |
919.9 |
930.9 |
929.8 |
899.7 |
Bloom characters
The interval of inflorescence development (from potassium chlorate application to flower emergence) of the August plot was shorter (53 days) than other plots (62 - 64 days). The development period until the end of blooming was also shorter in the August plot (61 days) (Table 4).
The sequence of blooming corresponded with potassium chlorate application, as did the start and end dates of blooming. Flowering in the August plot began (10% flowering) on the 278th Julian date and ended (100% flowering) on the 285th Julian date. Therefore, the August plot had the shortest total bloom period (8 days), and its full bloom day (60% flowering) happened on the 6th day of blooming (Table 4), while the flowering periods of the plots receiving early potassium chlorate application (May and June plots) were longer. Thus, flower development in longan trees receiving late potassium chlorate application (August) seemed to require a shorter amount of time to reach maturation in comparison to trees receiving earlier applications (May - June) (Table 1).
Sufficient water is necessary throughout the duration of longan growth and development. It requires an annual precipitation of at least 1,000 mm (Shi et al., 2015). The flower development of off-season longan throughout the annual rainy season might expose plants to slightly lower and unstable temperatures, as well as fluctuations in humidity. A relative humidity between 64% to 68% resulted in increased productivity, while higher relative humidity has been shown to lower the yield of on-season longan in Chiang Mai province, northern Thailand (Sritontip et al., 2014). Less water is needed in winter, and moderate drought is favorable for flower bud differentiation. (Shi et al., 2015).
Interestingly, the developmental performances of inflorescences appear to be associated with the minimum GDD of 1,096.4℃ in the August plot. Moreover, the average and daily minimum relative humidity in the August plot were relatively high (82.7% and 61.4%, respectively) (Table 3). However, the average maximum relative humidity of four studied plots; May, June, July, and August were similarly high of 96.9%, 97.3%, 96.9%, and 97.0%, respectively. Although the total amount of rainfall in the August plot was low (399.2 mm) (Table 3), days with high rainfall occurred just before and during blooming (2 - 3 October, 2020; 6 - 11 October, 2020) (Figure1). In Lampang province of northern Thailand, 32 - 63 mm of rainfall before the flower induction period in on-season longan increased fruit yield, whereas higher rainfall (64 - 128 mm) had the opposite effect (Sritontip et al., 2014). Excessive rain is unfavorable for longan flower and fruit development, as it can cause reduction in fruit-set rate and fruit quality (Shi et al., 2015). Thus, the microclimate of abundant rain in the central region of Thailand, as seen in Samut Sakhon province, is unsuitable for longan flower development.
Temperature is the most important factor that restricts the geographical distribution of longan. The preferred annual mean temperature for the cultivation of longan ranges from 20℃ to 22℃. (Shi et al., 2015). Due to the low GDD reflecting low air temperatures, the high humidity in the rainy season is often attributed to lower light intensity, which may be associated with a shorter period of flower development in the August plot. This means that late flower induction in August can lead to lower yield. Consequently, the low heat units and light intensity in the August plot may be related to frequent heavy rain during the blooming period (Figure 1). Lower temperatures correspond to lower GDD values and may result in late flowering since a high temperature is usually needed for flowering. Both excessively low and excessively high temperatures are known to influence fruit set (Shi et al., 2015). However, the response may vary depending on location and cultivar (Kanzaria et al., 2015). Shu (1999) noted that the biology of mangoes under different temperature regimes can vary. Flower duration under lower temperatures (19/13°C) can extend up to 36 days, while warmer temperatures (31/25°C) have been shown to decrease flower duration to 12 days. Therefore, various climate factors leading to high humidity and low light conditions can be an obstacle to off-season longan production. However, the effect of environment on longan flowering can vary. High rainfall and low relative humidity in Chiang Mai province resulted in increased productivity of ‘E-Daw’ longan, whereas in Lampang province, rainfall and relative humidity had the opposite effect (Sritontip et al., 2014).
Table 4. Bloom characters of off-season longan (Dimocarpus longan Lour. ‘Phuang Thong’) that developed at different times (May, June, July, and August) at Ban Phaeo district, Samut Sakhon province, Thailand in 2020.
|
Performance |
Plot name |
Significance |
||||
|
May |
June |
July |
August |
|
||
|
Period from potassium chlorate application to flower emergence (days) |
62 |
64 |
64 |
53 |
- |
|
|
Period from potassium chlorate application to bloom end (days) |
71 |
73 |
78 |
61 |
- |
|
|
Start of blooming (10% flowering) |
Jul. 21 |
Aug. 22 |
Sept. 20 |
Oct. 5 |
- |
|
|
(Julian date) |
(202nd) |
(234th) |
(263rd) |
(278th) |
|
|
|
End of blooming (100% flowering) |
Jul. 29 |
Aug. 30 |
Oct. 1 |
Oct. 12 |
- |
|
|
(Julian date) |
(210th) |
(242nd) |
(274th) |
(285th) |
|
|
|
Bloom period (days) |
9 |
9 |
12 |
8 |
- |
|
|
Day of full bloom (60% flowering) |
6.5 |
6.5 |
9.5 |
6 |
- |
|
|
Total bloom amount (number/inflorescence) |
716.5 ± 80.1 b |
1,412.4 ± 137.3 a |
665.5 ± 70.3 b |
925.6 ± 49.6 b |
* |
|
|
Percentage of female flowers (%) |
36.2 ± 4.1 a |
15.3 ± 2.0 b |
23.8 ± 2.9 b |
22.8 ± 3.1 b |
* |
|
|
Sex ratio (male:female) |
2:1 |
6:1 |
4:1 |
4:1 |
- |
|
Note: In each row, different means followed by the different letters represent significant differences (P ≤ 0.05), - indicates no statistical analysis.
The July plot had the longest bloom period with a significantly lower number of flowers (Table 4 and Figure 4). This pattern may have been caused by high rainfall during the floral developmental period, especially with heavy rain during blooming (Table 3 and Figure 1). The floral abundance of off-season longan during the rainy season is relatively low due to lower chemical efficacy caused by leaching (Manochai et al., 2005). Interestingly, most flowers in early potasium chlorate induced plots (May and June) opened on the first day, in contrast to the late induced plots (July and August) where most flowers opened on the 2nd - 3rd day (Figure 4). Flowers in the August plot showed the high bloom rate during days 2nd - 4th (Figure 4). As mentioned above, flowering in the August plot seemed to be influenced by the low heat unit or GDD.

Figure 4. Daily bloom number by Julian date in the rainy season of off-season longan (Dimocarpus longan Lour. ‘Phuang Thong’) produced at Ban Phaeo district, Samut Sakhon province, Thailand in 2020.
Remark: Arrow indicates of the potassium chlorate application of each plot.
Flower morphology
Various floral types were observed in the longan orchard at Ban Phaeo district, Samut Sakhon province, Thailand. During the rainy season, there were 3 types of off-season ‘Phuang Thong’ longan flowers: male or staminate or pistil-nonfunctional flowers (M1) (Figure 5A), functionally male hermaphrodite or fully hermaphrodite flowers (M2) (Figure 5B and 5C), and female or functionally female hermaphrodite or pistillate or stamens-nonfunctional flowers (F) (Figure 5D and 5E) (Choo, 2000; Pham et al., 2015a; Thu et al., 2017). Most male (M1) and female types (F) opened daily in all four plots. The M1 type was the main male type observed, while M2 was rarely found.

Figure 5. Types of flowers in off-season longan (Dimocarpus longan Lour. ‘Phuang Thong’) produced during the rainy season at Ban Phaeo district, Samut Sakhon province, Thailand in 2020. Photos show a male or staminate flower (A); functionally male hermaphrodite flowers (B and C); and female or functionally female hermaphrodite flowers (D and E)
The distribution pattern of female flowers was relatively comparable among all four plots. The number of male flowers was greater than that of female flowers (Figure 6). Interestingly, the number of female flowers in the July plot was higher than in the plots with later induction times (June, July and August) (Table 4). Heavy rain occurred during early flower development in the May plot, but then decreased during the blooming period (Figure 1). Such weather conditions in the May plot (Table 1) may promote large numbers of female flowers, and thus lead to high yield. These results correspond with those of Sritontip et al. (2014), who reported high yield following high rainfall (163 - 325 mm) in ‘E-Daw’ longan grown in Chiang Mai province in northern Thailand.

Figure 6. Daily bloom percentage of male (blue) and female (orange) flowers by Julian date during the rainy season of off-season longan (Dimocarpus longan Lour. ‘Phuang Thong’) at Ban Phaeo district, Samut Sakhon province, Thailand in 2020.
Remarks: Male refers to staminate flowers (type M1). Female refers to functioning female hermaphrodite flowers (type F).
Longan is a cross-pollinated plant with three types of flowers. In order to achieve cross-pollination, the flowers have to open sequentially, with a certain degree of overlap. Generally, within a panicle, the first phase in the blooming sequence is dominated by male flowers (M1), followed by functionally male hermaphrodite (M2) flowers, then by female flowers (F), and finally by male flowers (M1) a second time. The male and female phases of blooming overlap by 4 - 6 weeks depending on the cultivar (Choo, 2000). Longan in Vietnam was reported to have three indistinct waves of flowering. The first wave consisted of F flowers, the second of M2 flowers, and the third of M1 flowers. Usually, a few M2 flowers are also present in the first wave, whereas M1 flowers are abundant in the second wave, and a few F flowers are present in the third wave (Pham et al., 2013). This study found that the characteristics of flower opening were noticeably different among the three types. Staminate flowers (M1) and functionally female hermaphrodite flowers (F) bloomed together daily in the four studied plots with varying amounts. The flowering phases of these two flower types seem to have a short overlap between 8 - 12 days (Table 4). The bloom time of off-season ‘Phuang Thong’ longan in Samut Sakhon province in central Thailand is clearly different from the longer blooming period of on-season ‘Phuang Thong’ longan in Chiang Mai province in northern Thailand, which is usually around 36.5 days (Jantawan et al., 2016).
Additionally, the percentage of female blooms was the highest (36.2%) in the May plot, resulting in a low sex ratio of male to female flowers (2:1) (Table 4) in comparison to ‘E-Daw’ longan in Chiang Mai province (9.5:1) (Manochai et al., 2008). The three later induction times (June, July and August) showed the high male to female ratios of 6.1, 4:1, and 4:1, respectively (Table 4), which are similar to the on-season ‘Phuang Thong’ sex ratio at Chiang Mai province, Thailand (5.5:1) (Jantawan et al., 2016). Thus, sex expression of off-season ‘Phuang Thong’ longan exhibited different ratios among the four periods of potassium chlorate induction during the rainy season (Table 4). This result is in accordance with the remarkable differences observed between various seasons of ‘E-Daw’ longan in northern Thailand (Manochai et al., 2005). According to the findings of this study, the earlier flower induction time in May offered a more suitable environment for flower development, which resulted in longer inflorescences together with a higher percentage of female flowers, indicative of successful ‘Phuang Thong’ longan production in Samut Sakhon province, central Thailand.
In 2020, daily precipitation varied throughout the rainy season. During the late potassium chlorate application times of July and August (Table 1), rain was frequent, although rain amounts were similar. The number of flowers declined in such plots, and similar results were mentioned by Manochai et al. (2010). Potassium chlorate induction during the rainy season, especially whenever there is high rainfall during or after the application time, appears to have low effectiveness. The reason is likely caused by chemical leaching. High relative humidity could also limit the absorption of potassium chlorate due to low transpiration. Furthermore, the relatively low light intensity during the rainy season, due to both cloudy and rainy weather, could also influence flower development. Rainfall and light intensity during the critical induction period may affect flowering (Manochai et al., 2010). During the induction phase, between 10 - 15 days after potassium chlorate application, high rainfall and low light intensity might impact blooming. Weather also influenced potassium chlorate-mediated FI (flower induction) during the rainy season, which had the lowest flowering percentage, whereas cool and hot weather had the highest percentage (Manochai et al., 2005). Therefore, it can be concluded that longan flowering is reduced during the rainy season (Manochai et al., 2005; 2010; Sritontip et al., 2014). As a result, orchard microclimate clearly influences the bloom performance of off-season ‘Phuang Thong’ longan during the rainy season.
Successful fruit development depends largely on pollination and fertilization. Pollen germination and pollen viability are essential for effective cross-pollination. Various studies have reported that relative humidity and temperature can affect pollen performance. The pollen viability of Rosmarinus officinalis was enhanced by the co-occurrence of low temperatures and high humidity (Aronne et al., 2006). In contrast, Olea europaea L. exhibited lower pollen viability under conditions of high humidity and high temperature (Iovane et al., 2021).
This study evaluated the optimal conditions for in vitro pollen germination assessment in ‘Phuang Thong’ longan. Fresh pollen grains were collected and incubated at 30°C for 4 hours with a 15% sucrose concentration of germination media (Thu et al., 2017; Gupta et al., 2017). Results revealed that pollen from the August plot had the significantly highest germination rate of 80%, followed by the July plot (62%), the June plot (55%), and the May plot (43%) (Figure 7). The high pollen germination rate of the August plot was markedly associated with a low GDD of 1,096.4°C. The negative effects of high GDD are demonstrated by the reduced pollen fertility in the May, June, and July plots. The optimal temperature range for pollen germination in some species tends to be close to the average temperature of their flowering phenophase. A comprehensive analysis of the effect of temperature on pollen and ovule performance in ‘Brightwell’ blueberry showed that temperature significantly affected pollen germination, pollen tube growth, and ovule longevity. Moreover, pollen tube growth was accelerated by an appropriately high temperature range, resulting in a reduced travel time of the pollen tube to the ovule. The pollen tubes mostly stopped growing at around the middle of the style at 30°C, and no pollen tubes traversed the style at 35°C. The pollen tubes performed the best at temperatures of 15 - 25°C, suggesting that temperature range during the flowering phenophase can positively influence pollination, fertilization, and fruit setting (Yang et al., 2019). However, this study showed the high pollen viability rates of 95% in all four studied plots; May, June, July, and August of 97.6%, 94.5%, 95.7%, and 97.5%, respectively. In conclusion, pollen viability in off-season ‘Phuang Thong’ longan grown in central Thailand was not influenced by environmental factors.
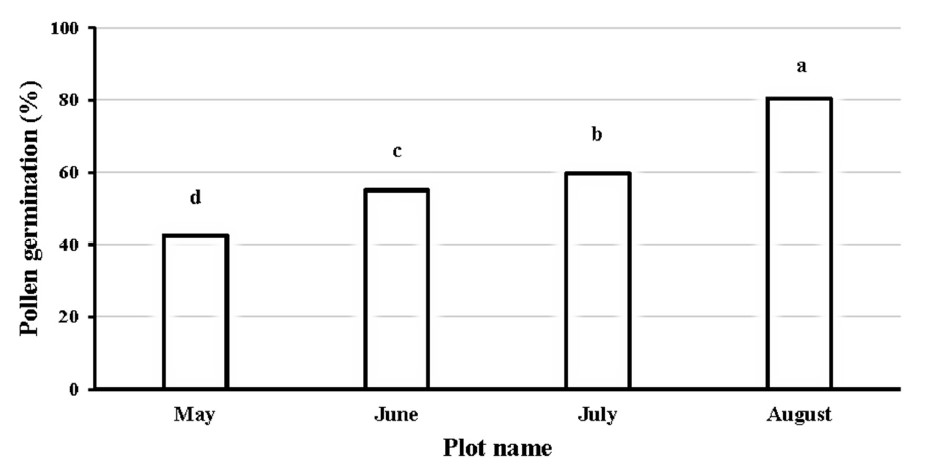
Figure 7. Percentage of pollen germination of off-season longan (Dimocarpus longan Lour. ‘Phuang Thong’) that developed at different times (May, June, July, and August) at Ban Phaeo district, Samut Sakhon province, Thailand in 2020.
Although the environmental conditions during flower development were different among the four plots, female flower performances in terms of size, stigma width, style length, ovary and ovule size were found to be comparable among the four induction times (Table 5). A comparison of ‘Phuang Thong’ longan grown in Samut Sakhon province and in Chiang Mai province revealed that the flower size found in Samut Sakhon province (7.09 - 7.37 mm) was larger than that found in Chiang Mai province (5.4 mm), but the style length was found to be shorter (4.85 - 5.74 mm) than those found in Chiang Mai province (6.4 mm) (Jantawan et al., 2016). The stigma width, ovary size and ovule size produced in Samut Sakhon province had a range of 1.22 - 1.47 mm, 2.03 - 2.21 mm, and 1.01 - 1.09 mm, respectively. It appears that variation in microclimate might also impact flower growth.
Table 5. The characters of female flower in off-season longan (Dimocarpus longan Lour. ‘Phuang Thong’) that developed at different times (May, June, July, and August) at Ban Phaeo district, Samut Sakhon province, Thailand in 2020.
|
Performance |
Plot name |
Significance |
|||
|
May |
June |
July |
August |
||
|
Flower size (mm) |
7.09 ± 0.08 |
7.37 ± 0.05 |
7.28 ± 0.06 |
7.24 ± 0.07 |
ns |
|
Stigma width (mm) |
1.29 ± 0.04 |
1.47 ± 0.05 |
1.34 ± 0.05 |
1.22 ± 0.04 |
ns |
|
Style length (mm) |
5.23 ± 0.08 |
5.74 ± 0.14 |
4.89 ± 0.07 |
4.85 ± 0.12 |
ns |
|
Ovary size (mm) |
2.05 ± 0.05 |
2.21 ± 0.05 |
2.14 ± 0.05 |
2.03 ± 0.05 |
ns |
|
Ovule size (mm) |
1.01 ± 0.02 |
1.06 ± 0.02 |
1.09 ± 0.03 |
1.06 ± 0.03 |
ns |
CONCLUSION
The potassium chlorate-induced off-season ‘Phuang Thong’ longan grown in Ban Phaeo district, Samut Sakhon province, central Thailand during the rainy season clearly showed differences in flowering and blooming characteristics among plots induced at different times: May, June, July, and August. The dissimilarity of bloom performance was associated with microclimate factors during inflorescence and flower development.
During the rainy season, flower development following early potassium chlorate application (May), which is expected to result in July blooming, yields longer inflorescences, but lower pollen germination. Two peaks of rainfall during the early period of flower development and during blooming might be suitable for better inflorescence growth and higher numbers of female flowers. In contrast, for late potassium chlorate application (August), which results in October blooming, flower development occurred during a period of low growing degree days and heavy frequent rainfall, which resulted in a short period of flower emergence and short inflorescence length. However, pollen viability and female flower performance were largely unaffected by environmental conditions during the rainy season. Microclimate components, particularly rainfall, temperature, and light intensity during flowering and inflorescence development, distinctly influenced the bloom performance of off-season ‘Phuang Thong’ longan production.
ACKNOWLEDGMENTS
We would like to acknowledge Thailand Science Research and Innovation for research funding (grant number RDG6120031) and the MUSC Scholarship for Human Resource Development in Science and Technology in Honor of the Late King Rama IX of Thailand, as well as Mahidol University for logistical support and facilities. We are grateful to Mr. Phairat Tienthong and the enterprise community members of longan farmers in Samut Sakhon province, Thailand for experimental fields and plant materials. We also thank Dr. Alyssa Stewart for assisting with English proofreading.
AUTHOR CONTRIBUTIONS
Conceptualization, methodology, formal analysis, investigation, resources, visualization, writing original draft preparation, Phyu Phyu Aung and Aussanee Pichakum; Data analysis and preparation, Phyu Phyu Aung, Nareenart Traisuwan, Tatpong Tulyananda and Watcharra Chintakovid; Review and editing the manuscript, Tatpong Tulyananda, Watcharra Chintakovid and Aussanee Pichakum. All authors have read and agreed to the published version of the manuscript.
CONFLICT OF INTEREST
The authors declare that they hold no competing interests.
REFERENCES
Aronne, G., De Micco, V., and Scala, M. 2006. Effects of relative humidity and temperature conditions on pollen fluorochromatic reaction of Rosmarinus officinalis L. (Lamiaceae). Protoplasma. 228: 127–130.
Choo, W.K. 2000. Longan production in Asia. Meetings and Publications Officer, FAO Regional Office for Asia and the Pacific, Bangkok, Thailand. RAP Publication: 2000/20. Series number: 1020-6221|1014-2789.
Gupta, A.K., Singh, M., Marboh, E.S., Nath, V., Pongener, A., and Anal, A.K.D. 2017. Pollen quantity, viability and in vitro pollen germination of longan (Dimocarpus longan Lour.). International Journal of Current Microbiology and Applied Sciences. 6(7): 270-278.
Iovane, M., Cirillo, A., Izzo, L.G., Di Vaio, C., and Aronne, G. 2021. High temperature and humidity affect pollen viability and longevity in Olea europaea L.. Agronomy. 12.(1):1. 10.3390/agronomy12010001.
Jantawan, W., Jaroenkit, T., Manochai, P., and Unpaprom., Y. 2016. Floral morphology of five longan cultivars (Dimocarpus longan Lour.) in Thailand. Srinakharinwirot Science Journal. 32(1): 131-141.
Jaroenkit, T., and Manochai, P. 2020. Current practices and research in off-season longan production in Thailand. Acta Horticulturae. 1293: 185-192.
Jaroenkit, T., Ussahatanonta, S., Thamjumrat, S., and Sritontip, C. 2014. Determination of longan (Dimocarpus longan 'Daw') baseline temperature in Thailand. Acta Horticulturae. 1029: 163-168.
Kanzaria, D., Chovatia, R., Varu, D., Polara, N., Chitroda, R., Patel, H., and Patel, D. 2015. Influence of growing degree days (GDD) on flowering and fruit set of some commercial mango varieties under varying climatic conditions. Asian Journal of Horticulture. 10(1): 130-133.
Manochai, P., Jaroenkit, T., Ussahatanonta, S., Ongprasert, S., and Kativat, B. 2010. Seasonal effect of potassium chlorate on flowering and yield of longan (Dimocarpus longan Lour.). Acta Horticulturae. 863: 363-366.
Manochai, P., Saritat, S., Suton, W., and Ussahatanonta, S. 2008. Effects of canopy height reduction on leaf flushing, flowering and yield of longan cv. E-Daw. The Journal of Agricultural Science. 39: 303–312.
Manochai, P., Sruamsiri, P., Wiriya-Alongkorn, W., Naphrom, D., Hegele, M., and Bangerth, F. 2005. Year around off season flower induction in longan (Dimocarpus longan Lour.) trees by potassium chlorate applications: Potentials and problems. Scientia Horticulturae. 104(4): 379-390.
Nacif, S.R., Sartori Paoli, A.A., and Chamhum Salomão, L.C. 2001. Morphological and anatomical development of the litchi fruit (Litchi chinensis Sonn. cv. Brewster). Fruits. 56(4): 225-233.
Pham, V.T., Herrero, M., and Hormaza, J.I. 2015a. Effect of temperature on pollen germination and pollen tube growth in longan (Dimocarpus longan Lour.). Scientia Horticulturae. 197: 470-475.
Pham, V.T., Herrero, M., and Hormaza, J.I. 2015b. Phenological growth stages of longan (Dimocarpus longan) according to the BBCH scale. Scientia Horticulturae. 189: 201-207.
Pham, V.T., Tran, M.H., Herrero, H., and Hormaza, J. 2013. The reproductive biology of the longan. p.1242-1246. In: Proceedings of the 5th National Scientific Conference on Ecology Biological Resources, 18 Oct 2013. Conference room of IEBR, Hanoi.
Pichakum, A., Traisuwan, N., Kammak, C., and Chintakovid, W. 2020. Climate change affecting off-season longan (Dimocarpus longan Lour.) production at alluvial plains of Thailand. Acta Horticulturae. 1293: 231-238.
Schneider, C.A., Rasband, W.S., and Eliceiri, K.W. 2012. NIH Image to ImageJ: 25 years of image analysis. Nature Methods. 9(7): 671-675.
Shi, S., Li, W., Zhang, H., Liu, L., Shu, B., Liang, Q., Xie, J., and Wei, Y. 2015. Application of extended Biologische Bundesantalt, Bundessortenamt and Chemische Industrie scale for phenological studies in longan (Dimocarpus longan). Annals of Applied Biology. 167: 127-134.
Shu, Z.S. 1999. Effect of temperature on the flowering biology and fertilization of mangoes (Mangifera indica L.). Journal of Applied Horticulture. 1(2): 79-83.
Sritontip, C., Jaroenkit, T., Manochai, P., and Sangchyoswat, C. 2014. The impact of climate changes on yield of longan production in northern Thailand. Acta Horticulturae. 1029: 155-162.
Subhadrabandhu, S., and Stern, R.A. 2005. Taxonomy, botany and plant development. p.25-34. In C.M. Menzel and G.K. Waite (eds) Litchi and longan: Botany, production and uses. CABI Publishing. Wallingford.
Subhadrabandhu, S., and Yapwattanaphun, C. 2000. Regulation of flowering time for longan (Dimorcarpus longan) production in Thailand. Journal of Applied Horticulture Lucknow. 2: 102-105.
Sukhvibul, N., Hetherington, S.E., Whiley, A.W., Smith, M.K., and Vithanage, V. 2000. Effect of temperature on inflorescence development and floral biology of mango (Mangifera indica L.). Acta Horticulturae. 509: 601-608.
Thu, M.K., Yuling, L., Jianjun, C., Chunzhen, C., Nigarish, M., Xuhan, X., and Zhongxiong, L. 2017. Flower types, pollen morphology, and in vitro pollen germination of longan (Dimocarpus longan Lour.). Journal of Botany Research. 1: 50-56.
Thunyarpar, T. 1998. Physiological aspects on flowering of lychee and longan: A review physiology of pollination and fertilization, for further development of horticulture in East Asia. Journal of the Japanese Society for Horticultural Science. 67(6): 1161-1163.
Yang, Q., Liu, E., Fu, Y., Yuan, F., Zhang, T., and Peng, S. 2019. High temperatures during flowering reduce fruit set in Rabbiteye blueberry. Journal of the American Society for Horticultural Science. 144(5): 339-351.
Zhou, B.Y., Chen, H.B., and Wu, C.B. 2014. An overview on natural triggers and stress signals in relation to flowering in Litchi chinensis and Dimocarpus longan. Acta Horticulturae. 1029: 137-144.
OPEN access freely available online
Natural and Life Sciences Communications
Chiang Mai University, Thailand. https://cmuj.cmu.ac.th
Phyu Phyu Aung1, 2, Nareenart Traisuwan1, 2, Watcharra Chintakovid3, Tatpong Tulyananda4, and Aussanee Pichakum1, *
1 Department of Plant Science, Faculty of Science, Mahidol University, Phayathai, Bangkok 10400 Thailand.
2 Department of Pharmaceutical Botany, Faculty of Pharmacy, Mahidol University, Phayathai, Bangkok 10400 Thailand.
3 Division of Agricultural Science, Mahidol University Kanchanaburi Campus, Sai Yok District, Kanchanaburi 71150 Thailand.
4 School of Bioinnovation and Bio-based Product Intelligence, Faculty of Science, Mahidol University, Phayathai, Bangkok 10400 Thailand.
Corresponding author: Aussanee Pichakum, E-mail: aussanee.pic@mahidol.ac.th
Total Article Views
Editor: Tonapha Pusadee,
Chiang Mai University, Thailand
Article history:
Received: April 10, 2023;
Revised: September 27, 2023;
Accepted: October 11, 2023;
Online First: October 30, 2023

