
Anticandidal Activity of Cajuput and Lemongrass Essential Oils Supplemented in Alcohol-Free Mouthwash Against Candida albicans Biofilm Formation on 96-Well Plate and Acrylic Surfaces
Rarinthorn Harintharanon, Chintana Itthidecharon, Phenphichar Wanachantararak, and Siriwoot Sookkhee*Published Date : October 10, 2023
DOI : https://doi.org/10.12982/NLSC.2023.067
Journal Issues : Number 4, October-December 2023
Abstract The present study aimed to evaluate the efficacy of alcohol-free mouthwash containing cajuput and lemongrass essential oils and their synergistic effect on eliminating Candida albicans biofilm formation, the most common causative agent of denture stomatitis. The inhibitory activity against C. albicans ATCC10231 biofilm formation on 96-well plate and acrylic surfaces of this formula was significantly different from 0.12% chlorhexidine solution at 20 minutes (P < 0.0001). At one hour and eight hours of immersion, the activity of this formula was similar to the activity of 0.12% chlorhexidine mouthwash. After treatment with this formula, there were less densely active cells and biofilm compared to the negative control, and the action was close to that of 0.12% chlorhexidine mouthwash. The minimal inhibitory concentrations of cajuput and lemongrass essential oils were 2 and 4 µL/mL (for 0.2% and 0.4% v/v, respectively). For chequerboard assays, the fractional inhibitory concentrations of these oils were 0.5 and 0.25 µL/mL, respectively. The combination of cajuput and lemongrass oils in this formula exhibited partial synergism against C. albicans ATCC10231 biofilm formation with a fractional inhibitory concentration index of 0.75. This study demonstrated the inhibitory activity of this formula against C. albicans biofilm formation on 96-well plate and acrylic surfaces after quantitated by colony enumeration and the XTT reduction assay resembled 0.12% chlorhexidine mouthwash. In conclusion, that C. albicans could be inhibited by the partial synergism of these essential oils in this mouthwash formula.
Keywords: Inhibitory activity, Natural essential oils, Candida albicans, Microbial biofilm, Solid surface
Funding: We are grateful for the research funding provided by the Faculty of Dentistry, Chiang Mai University, Chiang Mai, Thailand.
Citation: Harintharanon, R., Itthidecharon, C., Wanachantararak, P., and Sookkhee, S. 2023. Anticandidal activity of cajuput and lemongrass essential oils supplemented in alcohol-free mouthwash against Candida albicans biofilm formation on 96-well plate and acrylic surfaces. Natural and Life Sciences Communications. 22(4): e2023067.
INTRODUCTION
Denture stomatitis is the most common symptom in the elderly who has worn ill-fitting dentures and has inappropriate oral care leading to candidal infection (Gendreau and Loewy, 2011). It presents as erythematous lesions indicating inflammation at the hard palate and edentulous area under the denture. It also presents a white lesion that indicates a pathologic lesion from Candida albicans. Candida albicans is an important oral dimorphic fungal flora. It can adhere to various surfaces including enamel, soft tissues, and dentures. However, it can be pathogenic when the host suffers from predisposing factors. Its lesions can be found in oral soft tissues. It is also found in the form of biofilm at acrylic surface (Naik and Pai, 2011). As the aging population of over 60 years old increases to up to 20%, the 8th national oral health survey of Thailand in A.D. 2017 reported that the incidence of partial removable and complete denture wearing in 60, 74, 80 and 85 years old elderly were 18%, 10.5%, 15.3% and 27.4%, respectively (Khiddee et al., 2018). Therefore, dentists should be aware of the problems of denture wearing that has increase in the elderly (Cumming et al., 1990). The combination of mechanical cleaning and chemical cleaning is the recommended method to clean dentures. Mechanical cleaning such as brushing is commonly used in a routine of people for eliminating denture plaque. Apart from mechanical cleaning, chemical cleaning is recommended to enhance the protective activity for microbial pathogens. The examples of chemical cleaning are mouthwash and denture cleanser. Commonly, 0.12% chlorohexidine mouthwash is the most widely used chemical cleanser as the antiseptic mouthwash and denture-immersed solution. It can effectively eliminate microbial plaque with side effects of staining of dentures and the alteration of tasting. Alternatively, natural products and its essential oils have been known to exhibit antimicrobial activity to inhibit the pathogens. The interest of this study focused on the inhibitory activity of microbial biofilm in oral cavity by the essential oil supplementation in mouthwash. Cajuput and lemongrass essential oils, extracted from Melaleuca cajuputi Powell. and Cymbopogon citratus, respectively, have already been used as medical antiseptic agents (Gao et al., 2020; Jedlicková et al., 1992; Taweechaisupapong et al., 2012a). The individual and combined essential oils can inhibit some Candida species, C. albicans, C. vaginalis and C. glabrata at 0.4-0.6% cajuput oil (Keereedach et al., 2020); C. albicans, C. glabrata, C. krusei, C. parapsilosis and C. tropicalis with 4 and 8 µL/disc (Freire et al., 2017), and C. albicans, with essential oil supplementation (8 µL of cajuput oil and 4 µL of lemongrass oil/mL) in alcohol-free mouthwash (AM). The latter combination gave better inhibitory effect of 80% (Raungsawat et al., 2019). Candida albicans biofilm formation on solid surfaces resisted to chemical cleaning and caused physical denture injury (Gulati and Nobile, 2016). Inhibitory activity of lemongrass essential oil against C. albicans biofilms on acrylic surface has previously been studied, but there is no report on cajuput essential oil. The purpose of this study was to determine anticandidal activity of cajuput and lemongrass essential oil supplemented in alcohol-free mouthwash (AM) against C. albicans biofilm on acrylic surface. These can be used as alternative mouthwash for denture immersion to prevent denture stomatitis.
MATERIALS AND METHODS
Preparation of essential oils
Natural essential oils were extracted from leaves of Melaleuca cajuputi Powell (Southern Thailand) and Cymbopogon citratus (Chiang Mai, Thailand). Five kilograms of each fresh leaves were prepared in distilled water using Soxhlet extractor. The obtaining yellow-green cajuput and yellow-orange lemongrass essential oils were stored in sterile bottles wrapped in aluminum foil, protecting from the light, and then refrigerated.
Preparation of cajuput and lemongrass oils supplemented in alcohol-free mouthwash (CLAM)
One milliliter of cajuput and lemongrass oils supplemented in alcohol-free mouthwash (CLAM) consisted of 618 µL distilled water, 120 µL propylene glycol (12% v/v), 50 µL sorbitol (70% solution, 5% v/v), 100 µL menthol (0.4 mg/mL solution), 100 µL sodium benzoate (one mg/mL solution), 8 µL cajuput essential oil and 4 µL of lemongrass essential oil (Costa et al., 2013; Raungsawat et al., 2019).
Preparation of saliva
Saliva was collected from healthy volunteers, with no medical history or medication affecting on saliva flow, in the morning by splitting method. Food and drink fasting and oral cavity cleaning were requested before sampling. Voluntarily was approved by the Human Experimentation Committee, Faculty of Dentistry, Chiang Mai University (No. 48/2021). Salivary sample was centrifuged at 10,000 rpm, 4 °C for 30 min, and the supernatant was then filtered by 0.45 μm sterilized membrane (PuridiscTM 25 mm, GE Healthcare UK Ltd., Buckinghamshire, UK). One hundred μL of each sterilized saliva were pipetted into 96-well plate and aerobically incubated at 37 °C for 2 h. Salivary pellicle was washed twice and resuspended with 200 μL of phosphate buffer saline (PBS) solution. One hundred μL of sterile saliva was spread on Sabouraud Dextrose Agars (SDA; Merck KGaA, Darmstadt, Germany) and incubated at 37°C for 24 h.
Candida albicans strains
C. albicans ATCC10231 was provided from Thailand Institute of Scientific and Technological Research (TISTR), Thailand. These clinical strains of C. albicans were isolated from the patients diagnosed with oral candidiasis at the Oral Diagnosis Clinic and confirmed as C. albicans on HiCromeTM Candida Differential agar (Himedia, Mumbai, India). Three C. albicans strains were cultured in Sabouraud Dextrose Broth (SDB; Merck KGaA, Darmstadt, Germany) and aerobically incubated at 37 °C for 16-18 h. One milliliter of C. albicans culture was centrifuged at 1,000 rpm for 5 min. Cell pellet was resuspended in 1 mL SDB and optical density at 600 nm (OD600nm) was adjusted to be equal to 0.5 McFarland standard (1x106 CFU/mL).
Preparation of C. albicans biofilms on 96-well plate
One hundred µL of C. albicans suspension adjusted to be equal to 0.5 McFarland standard was pipetted into 96-well plate and aerobically incubated at 37 °C for 1.5 h. One hundred µL of SDB was used as control (Costa et al., 2013). Cell pellicle was washed twice with 200 µL PBS solution. During 48 h incubation, 200 µL SDB was also changed every 24 h. Finally, C. albicans biofilm was washed twice with 200 µL PBS solution before use in further experiments.
Inhibitory activity of CLAM against C. albicans biofilm formation on surface of 96-well plate using colony enumeration
To determine the inhibitory activity of cajuput and lemongrass essential oils supplemented in alcohol-free mouthwash (CLAM) in 96-well plate, 200 µL CLAM was pipetted into the biofilm coated 96-well plate. After 20 min, 1 h and 8 h incubation at 37 °C, biofilm was washed twice and resuspended in 200 and 100 µL PBS solution, respectively. Ten-fold dilution was made from whole cell suspension using PBS solution, finishing at 10-3 dilution (Madeira et al., 2016). One hundred µL of each dilution was spread on SDA plates. Plates were aerobically incubated at 37 °C for 24 h. Sabouraud Dextrose Broth (SDB) and 0.12% chlorhexidine (CHX) mouthwash (C-20 red, Osoth Inter Laboratories Co. Ltd, Bangkok, Thailand) were used as the negative and positive controls, respectively. The candida colonies were enumerated and converted into CFU/mL. Percentage of inhibitory activity was calculated as follows (Khalilzadeh et al., 2010).
% inhibitory activity = CFU/mL of untreated – CFU/mL of treated X 100
CFU/ml of untreated
Inhibitory activity of CLAM against C. albicans biofilm formation on 96-well plate using fluorescence microscopy
To determine the inhibitory activity of CLAM in 96-well plate, 200 µL CLAM was pipetted into the biofilm coated 96-well plate. After 8 h incubation at 37 °C, biofilm was washed twice with 200 µL PBS solution and stained with 5 µM/mL of 2-chloro-4-(2,3-dihydro-3-methyl-(benzo-1,3-thiazol-2-yl)-methylidene)-1-phenyl quinolinium iodide (FUN-1) in PBS solution, and 25 μg/mL of Alexa Fluor 488 conjugate of concanavalin A (ConA-Alexa Fluor 488) (InvitrogenTM, Thermo Fisher Scientific, Waltham, MA). Plate was wrapped with aluminum foil, protecting from the light, at 37 °C for 40 min (Curvelo et al., 2019). Yeast cells were observed by fluorescent microscopy (CCD Microscope Camera Leica DFC3000G, Leica, Wetzlar, Germany) at 400x magnification.
Fabrication of acrylic discs
A total of 300 round-shaped heat-polymerized acrylic disc (PMMA, 6 mm diameter, 2 mm thickness) was fabricated by a dental technician at Hexa Ceram Co., Ltd, Chiang Mai, Thailand (Lot No. REA0121). They were immersed in distilled water at 37 °C for 48 h, and subsequently sterilized before using (Paranhos et al., 2009).
Preparation of C. albicans biofilm on acrylic surface
Individual acrylic disc was vertically placed in each well of 96-well plate. Two hundred μL of sterilized saliva were dropped on each acrylic disc, and aerobically incubated at 37 °C for 2 h. Salivary pellicle was washed twice with 200 μL of PBS solution. Two hundred μL of C. albicans cultured in SDB, with OD600 nm equal to 0.5 McFarland standard, were added and aerobically incubated at 37 °C for 1.5 h. Acrylic disc was washed twice with 200 µL of PBS solution. During 48 h incubation, 200 μL SDB was also changed every 24 h (Choonharuangdej et al., 2021). Finally, C. albicans biofilm was washed twice with 200 µL PBS solution before use in further experiment.
Inhibitory activity of CLAM against C. albicans biofilm formation on acrylic surface using colony enumeration
To determine the inhibitory activity of CLAM on acrylic surface, 200 µL CLAM was dropped on the biofilm coated acrylic discs. After 20 min, 1 h and 8 h incubation at 37 °C, biofilm was washed twice in 200 µL of PBS solution. Acrylic disc was immersed in 1 mL SDB and agitated for 15 min by BioSonic ultrasonic cleaner (BioSonic® UC125, COLTENE, West Sussex, UK). Ten-fold dilution was made from whole cell suspension using PBS solution, finishing at 10-2 dilution (Siriyod et al., 2023). One hundred µL of each dilution was spread on SDA. Plates were aerobically incubated at 37°C for 24 h. SDB and 0.12% CHX mouthwash were used as negative and positive controls, respectively. Percentage of inhibitory activity was calculated as previously described.
Inhibitory activity of CLAM against C. albicans biofilm formation on acrylic surface using xtt reduction assay
The solution of sodium 3’-[1-(phenylaminocarbonyl)-3,4-tetrazolium]-bis(4-methoxy-6-nitro)benzene sulfonic acid hydrate (XTT) was freshly prepared as follows. XTT powder (Thermo Fisher Scientific, Waltham, MA.) was dissolved in PBS solution (0.5 mg/mL), while phenazine methosulphate powder (Sigma-Aldrich, St. Louise, MO.) was prepared in distilled water (0.32 mg/mL). Finally, the two solutions were mixed at the ratio of 9:1 respectively (Gulati et al., 2018). To determine the inhibitory activity of CLAM on acrylic surface, 200 µL CLAM was dropped on the biofilm coated acrylic discs. After 20 min, 1 h and 8 h incubation at 37 °C, biofilm was washed twice in 200 µL PBS solution.
Two hundred µL of XTT solution were dropped on each acrylic disc. Plate was wrapped in aluminum foil, protecting from the light, and aerobically incubated at 37 °C for 3 h. Reaction mixture was then measured at OD490nm using the Sunrise Tecan® absorbance microplate reader (Tecan Group Ltd., Männedorf, Switzerland). Percentage of inhibitory activity was calculated as follows.
% inhibitory activity of mouthwash = 1 – OD492 of treated X 100
OD492 of untreated
Determination of minimum inhibitory concentrations (MIC) and fractional inhibitory concentration index (FICI) of CLAM against C. albicans
Minimum inhibitory concentrations (MIC) of each natural essential oil was determined by broth microdilution (two-fold dilution), with AM at the final concentration ranging from 0.125 - 16 μL/mL. CHX mouthwash and AM were serially two-fold diluted with SDB, at the final concentration ranging from 0.001875% - 0.48% (v/v). Fifty µL of C. albicans suspension adjusted to be equal to 0.5 McFarland standard were pipetted into 96-well plate and aerobically incubated at 37 °C for 24 h. Fifty µL of SDB were used as a control. After 24 h, MIC was defined as the lowest concentration of essential oils that could inhibit the growth of C. albicans.
Fractional inhibitory concentration (FIC) of each natural oil was performed by checkerboard assay (Sookkhee et al., 2022). Two-fold serial dilutions, with the concentration ranging from 0.25-16 µL/mL, were done in column 1 to 7 and row A to G, for cajuput and lemongrass essential oils, respectively. Cajuput and lemongrass essential oils alone were also serial diluted in column 8, and row H, respectively. Fifty µL of C. albicans suspension adjusted to be equal to 0.5 McFarland standard were pipetted into 96-well plate and aerobically incubated at 37 °C for 24 h. Fifty µL of two natural essential oils were used as the controls. After 24 h incubation, FICI was interpreted as a value representing a synergistic, additive, indifferent, or antagonistic effect, employing the following formula (Sookkhee et al., 2022),
FICCajuput Oil = (MICCajuput Oil in combination)/(MICCajuput Oil alone)
FICLemongrass Oil = (MICLemongrass Oil in combination)/(MICLemongrass Oil alone)
FICI = FICCajuput Oil + FICLemongrass Oil
FICI ≤ 0.50 denoting synergism
0.50 < FICI ≤ 0.75 denoting partial synergy
0.75 < FICI ≤ 1 denoting an additive effect
1 < FICI ≤ 4 denoting indifference or no interaction
and FICI > 4 denoting antagonism
RESULTS
Inhibitory activity of CLAM against C. albicans biofilm formation on 96-well plate using colony enumeration
Inhibitory activity of CLAM was significantly different from those of AM at 20 min, 1 h and 8 h for all C. albicans isolates (ATCC10231, patient 1 and patient 2) at P < 0.0001. A significant difference was also observed in CLAM and 0.12% CHX solution at 20 min, although there was no the significant difference between CLAM and 0.12% CHX solution at 1 h and 8 h for all yeasts at P < 0.0001 (Figure 1). At 1 h incubation in CLAM, both two clinical strains showed a significant difference from C. albicans ATCC10231 at P < 0.01 (data not shown).

Figure 1. Inhibitory activity of AM (spot bar), CLAM (diagonal bar) and 0.12% CHX solution (grid bar) against C.albicans biofilm formation in 96-well plate at 20 min, 1 h and 8 h incubation using colony enumeration. C. albicans strains (A) ATCC10231; (B) patient 1; (C) patient 2. Significant difference at *, P ≤ 0.001. **, P ≤ 0.0001.
Inhibitory activity of CLAM against C. albicans ATCC10231 biofilm formation on 96-well plate using fluorescence microscopy
Fluorescence images (400x magnification) of C. albicans ATCC10231 biofilm formation in 96-well plate at 8 h incubation in SDB, AM, CLAM and 0.12% CHX solution revealed living cells and extracellular matrix stained in red (left column) and light green (middle column), respectively. A merge of the two columns was shown in yellow (right column), indicating that living cells could produce extracellular matrix and subsequently formed biofilm (Figure 2). Living cell number and extracellular matrix content after treatment with CLAM tended to be less dense in comparison with SDB and AM. While a similar result was observed in 0.12% CHX solution, suggesting that CLAM could inhibit growth of C. albicans and its biofilm formation.

Figure 2. Fluorescent images (400x magnification) of C. albicans ATCC10231 biofilm formation, staining with FUN-1 (left column), ConA-Alexa Fluor (middle column) and merging two column (right column) after 8 h incubation in SDB, AM, CLAM, and 0.12% CHX solution.
Inhibitory activity of CLAM against C. albicans biofilm formation on acrylic surface using colony enumeration
Inhibitory activity of CLAM was significant different from those of AM at 20 min, 1 h and 8 h for all C. albicans isolates (ATCC10231, patient 1 and patient 2) at P ≤ 0.0001. A significant difference was also observed in CLAM and 0.12% CHX solution at 20 min and 1 h, although there was no the significant difference between CLAM and 0.12% CHX solution at 8 h for all tested isolates at P ≤ 0.0001 (Figure 3). At 8 h incubation in CLAM, both two clinical strains showed a significant difference from C. albicans ATCC10231 at P ≤ 0.001 (data not shown).
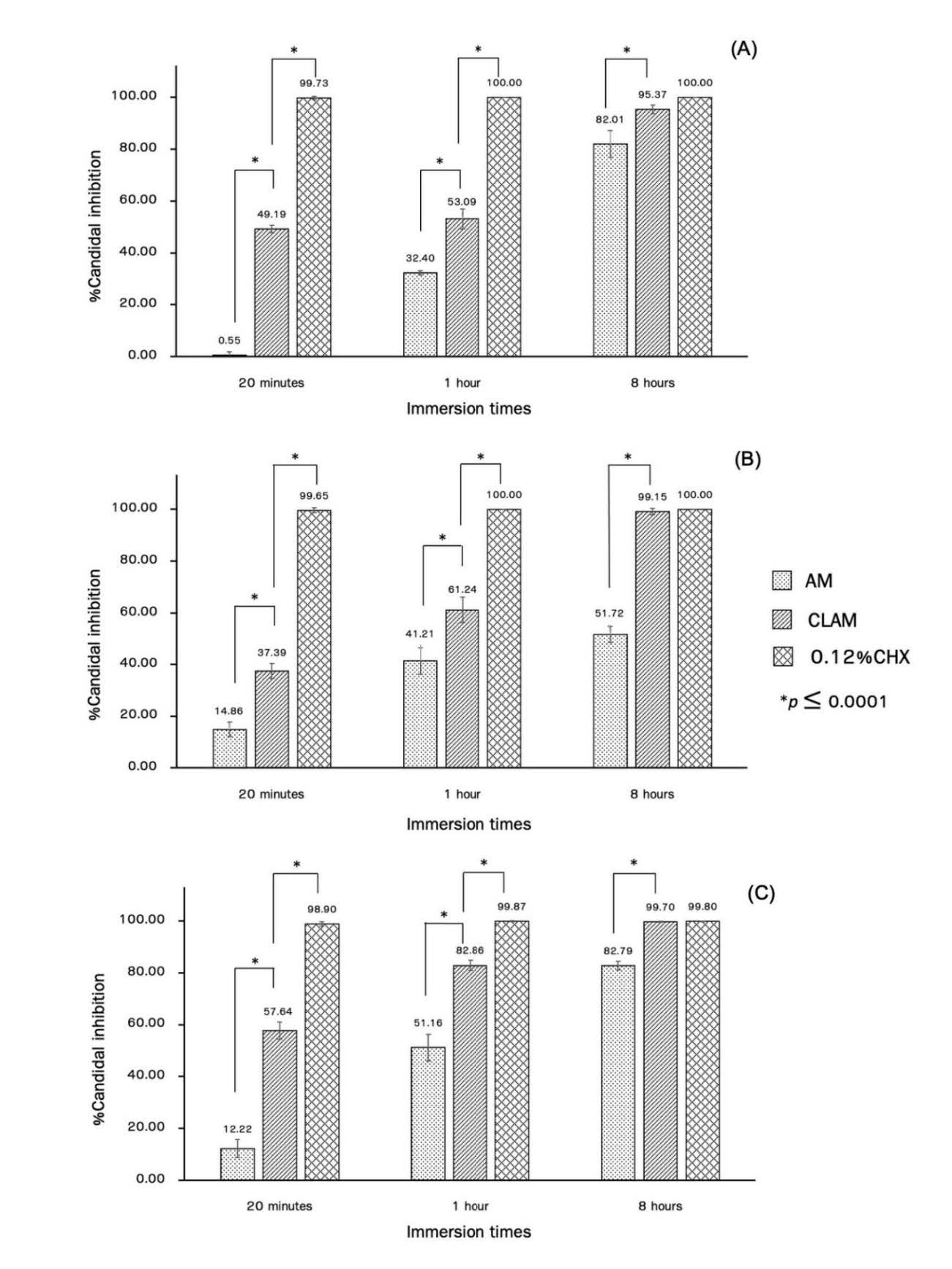
Figure 3. Inhibitory activity of AM (spot bar), CLAM (diagonal bar) and 0.12% CHX solution (grid bar) against C. albicans biofilm formation on acrylic surface at 20 min, 1 h and 8 h incubation using colony enumeration. C. albicans (A) ATCC10231; (B) patient 1; (C) patient 2. Significant difference at *, P ≤ 0.0001.
Inhibitory activity of CLAM against C. albicans biofilm formation on acrylic surface using xtt reduction assay.
Inhibitory activity of CLAM was significantly different from those of AM at 20 min, 1 h and 8 h for all tested C. albicans isolates at P ≤ 0.0001. A significant difference was also observed in CLAM and 0.12% CHX solution at 20 min and 1 h, although there was no the significant difference between CLAM and 0.12% CHX solution at 8 h for all tested isolates at P <0.0001 and 0.01 (Figure 4). At 8 h incubation in CLAM, both two clinical strains showed a significant difference from C. albicans ATCC10231 at P ≤0.05 (data not shown).

Figure 4. Inhibitory activity of AM (spot bar), CLAM (diagonal bar) and 0.12% CHX solutions (grid bar) against C. albicans biofilm formation on the acrylic surface at 20 min, 1 h and 8 h incubation using XTT reduction assay. C. albicans (A) ATCC10231; (B) patient 1; (C) patient 2. Significant difference at *, P ≤ 0.05; **, P ≤ 0.01, ***, P ≤ 0.001; ****, P ≤ 0.0001.
Determination of the minimum inhibitory concentration (MIC) and the fractional inhibitory concentration index (FICI) of CLAM against C. albicans
All tested concentrations of CHX mouthwash (0.001875-0.48%) could completely inhibit C. albicans ATCC10231. Minimal inhibitory concentration (MIC) of cajuput and lemongrass essential oils was 2 and 4 µL/mL, respectively, as shown in Table 1. Fractional inhibitory concentration (FIC) of these two essential oils (in combination) was obtained at 0.5 and 0.25 µL/mL, respectively. The calculated index of FIC (FICI) was 0.75, interpreting that C. albicans could be inhibited by the partial synergic effect of natural essential oils in CLAM.
Table 1. Minimal inhibitory concentration (MIC), fractional inhibitory concentration (FIC) and calculated index of FIC (FICI) of two essential oils (alone and in combination) against C. albicans ATCC10231.
|
Essential oils |
MIC (µL/mL) |
FIC |
FICI |
Interpretation |
|
|
alone |
combination |
|
|
|
|
|
Cajuput |
2 |
1 |
0.5 |
0.75 |
Partial synergy |
|
Lemongrass |
4 |
1 |
0.25 |
||
DISCUSSION
The present study focused on the anticandidal activity of CLAM in emulsion formulation Chanpa et al. reported that the emulsion formulation was affected by three important compositions i.e. oil phase, emulsifier and distilled water (Chanpa et al., 2023). Propylene glycol was used as an emulsifier in CLAM. Acrylic denture base, usually made from polymethylmethacrylate (PMMA), was highly adhered by C. albicans due to its micropit and microsporosity. Surface roughness might encourage microbial retention and infection (Hasan and Singh, 2015). C. albicans colonized on acrylic surfaces and formed biofilm appeared to resist to disinfectant and antifungal agent. Therefore, inadequate cleaning was a crucial factor for rapidly pathogenic biofilm adhesion and denture plaque accumulation (Gendreau and Loewy, 2011). In addition, proper cleansing was also important for denture sterility before use, Thai cajuput oil has inhibitory activity against C. albicans (Keereedach et al., 2020). Taweechaisupapong et al. have reported that lemongrass oil exhibited antifungal and antibiofilm activities on clinical C. albicans (Taweechaisupapong et al., 2012a; Taweechaisupapong et al., 2012b). Mouthwash formulated with cajuput (8 μL/mL) and lemongrass (4 μL/mL) essential oils showed relatively stronger inhibitory effect (> than 80%) against the C. albicans planktonic cells (Raungsawat et al., 2019). There was no previous report on solid surfaces, and thus the anticandidal activity of cajuput and lemongrass essential oils in CLAM against C. albicans biofilm formation in 96-well plate and acrylic surface was investigated as well as MIC and FICI of these two essential oils in combination was also evaluated with some modification of those from previous reports (Madeira et al., 2016; Choonharuangdej et al., 2021). Combination of cajuput and lemongrass essential oils showed a partial synergic effect against C. albicans ATCC10231 (FICI = 0.75), which was comparable to value of 0.58 (Ishijima et al., 2021). Whereas, combination of lemongrass essential oil and CHX mouthwash gave a synergic effect at value of 0.31 (Satthanakul et al., 2019). Inhibitory activity of cajuput and lemongrass essential oils against clinical Candida isolates ranged from 0.4-0.6% (v/v) (Chao et al., 2000; Wińska et al., 2019) and 0.32% -1% (v/v) (Koseki et al., 2018; Choonharuangdej et al., 2021), respectively. Anticandidal activity in solid surfaces was time-dependent as the highest efficacy was similar to those of 0.12% CHX mouthwash, a positive control, at 8 h incubation. However, lower anticandidal activity on acrylic surface was observed in comparison with 96-well plate, possibly due to micropit and microporosity of acrylic surface. The efficacy was confirmed by fluorescent microscopy, revealing metabolic living cells and extracellular matrix production. Similar result was also observed in fluorescent image analysis at 8 h incubation, indicating that this was correlated with time. According to XTT reduction assay (Gulati et al., 2018), cellular viability and metabolic activity was measured by colorimetric spectrophotometry. It was found that the highest efficacy was observed at 8 h incubation in contrast to a previous report (Choonharuangdej et al., 2021), indicating that different compositions and appropriate formulations of mouthwash could result in high stability.
Main component of cajuput and lemongrass essential oils were citral and 1, 8-cineole, respectively (Chao et al., 2000). Citral exhibited inhibitory activity on C. albicans and C. krusei biofilms (Taweechaisupapong et al., 2012a), whereas 1, 8-cineole possessed anticandidal and synergic activity with chlorhexidine digluconate (Hendry et al., 2009). Increasing of lemongrass essential oil concentration exhibited better efficacy as described by Ambade et al (Ambade et al., 2022). Therefore, physical properties (color, odor, taste, phase and transparency) of mouthwash will be further examined. CLAM might be used as alternative option for denture cleansing in combination with brushing for plaque removal. However, emulsion stability (oil-water) and pleasant sensation (taste, color, smell) would be concerned for further clinical trials.
CONCLUSION
C. albicans is a most common oral pathogen that causes denture stomatitis in the elderly. Novel therapeutic approaches for fungal eradication are required as several factors, including immunocompromised condition, ineffective mouthwash and denture cleanser, and antifungal drug resistance. Two essential oils, lemongrass oil and cajuput oil, in combination was developed for natural alcohol-free mouthwash. Inhibitory activity against C. albicans biofilm formation in 96-well plate and acrylic surface was observed, showing a partial synergism. Novel natural mouthwash might be used as an alternative option for growth inhibition, biofilm formation and pathogenic infection of denture stomatitis.
ACKNOWLEDGMENTS
The authors gratefully acknowledge Dr. Thanapat Sastraruji, Dental Research Center, Faculty of Dentistry, Chiang Mai University for statistical analyses. We also thank the technicians and all volunteers at the Comprehensive Dental Clinic, Faculty of Dentistry. This research would have been impossible without the approval of the Faculty of Dentistry Human Experimentation Committee, Faculty of Dentistry, Chiang Mai University. This study received approval from the Institutional Biosafety Committee of Chiang Mai University, code number CMUIBC A-0565003.
AUTHOR CONTRIBUTIONS
Rarinthorn Harintharanon wrote the research proposal, prepared the IBC document, designed and did the experiments, and wrote the manuscript draft. Chintana Itthidecharon provided the conceptualization, consulted on the research proposal, and proofread the research work. Phenphichar Wanachantararak designed the experiments, consulted on the IBC document, provided the resources, consulted on the experiments. Siriwoot Sookkhee consulted on the research proposal, analyzed the statistical data, wrote the manuscript and proofread the research work. All authors have read and approved the final published version of manuscript.
CONFLICT OF INTEREST
No potential conflicts of interest relevant to this article were reported.
REFERENCES
Ambade, S., Deshpande, N., Abhyankar, P. 2022. Effect of lemongrass essential oil based mouthwash against microflora associated with dental plaque. Journal of Pure and Applied Microbiology. 16(1): 174-181.
Chanpa, P., Owittayakul, D., Wanachantararak, P., Chaiyana, W., Sookkhee, S. 2023. Formulation of coconut oil mouthwash with mixed emulsifier and its growth inhibition of Candida albicans biofilms. Natural and Life Sciences Communications. 22(1): 1-16.
Chao, S. C., Gary Young, D., Oberg, C. J. 2000. Screening for inhibitory aactivity of essential oils on selected bacteria, fungi and viruses. Journal of Essential Oil Research. 12(5): 639-649.
Choonharuangdej, S., Srithavaj, T., Thummawanit, S. 2021. Fungicidal and inhibitory efficacy of cinnamon and lemongrass essential oils on Candida albicans biofilm established on acrylic resin: An in vitro study. Journal of Prosthetic Dentistry. 125(4): 707.e701-707.e706.
Costa, A. C., Pereira, C. A., Freire, F., Junqueira, J. C., Jorge, A. O. 2013. Methods for obtaining reliable and reproducible results in studies of Candida biofilms formed in vitro. Mycoses. 56(6): 614-622.
Cumming, C. G., Wight, C., Blackwell, C. L., Wray, D. 1990. Denture stomatitis in the elderly. Oral Microbiology and Immunology. 5(2): 82-85.
Curvelo, J. A. R., Moraes, D. C., Anjos, C. A. D., Portela, M. B., Soares, R. M. A. 2019. Histatin 5 and human lactoferrin inhibit biofilm formation of a fluconazole resistant Candida albicans clinical isolate. Anais da Academia Brasileira de Ciências. 91(1): e20180045.
Freire, J. C. P., Júnior, J. K. O., Silva, D. F., de Sousa, J. P., Guerra, F. Q. S., de Oliveira Lima, E. 2017. Antifungal activity of essential oils against Candida albicans strains isolated from users of dental prostheses. Evidence-Based Complementary and Alternative Medicine. 2017: 7158756.
Gao, S., Liu, G., Li, J., Chen, J., Li, L., Li, Z., et al. 2020. Antimicrobial activity of lemongrass essential oil (Cymbopogon flexuosus) and its active component citral against dual-species biofilms of Staphylococcus aureus and Candida species. Frontier in Cellular and Infection Microbiology. 10: 603858.
Gendreau, L. and Loewy, Z. G. 2011. Epidemiology and etiology of denture stomatitis. Journal of Prosthodontics. 20: 251-260.
Gulati, M., Lohse, M. B., Ennis, C. L., Gonzalez, R. E., Perry, A. M., Bapat, P., et al. 2018. In vitro culturing and screening of Candida albicans biofilms. Current Protocals in Microbiology. 50(1): e60.
Gulati, M. and Nobile, C. J. 2016. Candida albicans biofilms: Development, regulation, and molecular mechanisms. Microbes and Infection. 18(5): 310-321.
Hasan, S. and Singh, K. 2015. Denture stomatitis: A literature review. Journal of Orofacial & Health Sciences. 6(2): 65-69.
Hendry, E. R., Worthington, T., Conway, B. R., Lambert, P. A. 2009. Antimicrobial efficacy of eucalyptus oil and 1,8-cineole alone and in combination with chlorhexidine digluconate against microorganisms grown in planktonic and biofilm cultures. Journal of Antimicrobial Chemotherapy. 64(6): 1219-1225.
Ishijima, S. A., Ezawa, K., Abe, S. 2021. Lemongrass and perilla essential oils synergistically increased antimicrobial activity. Medical Mycology Journal. 62(4): 79-87.
Jedlicková, Z., Mottl, O., Serý, V. 1992. Antibacterial properties of the Vietnamese cajeput oil and ocimum oil in combination with antibacterial agents. Journal of Hygiene Epidemiology, Microbiology, and Immunology. 36(3): 303-309.
Keereedach, P., Hrimpeng, K., Boonbumrung, K. 2020. Antifungal activity of Thai cajuput oil and its effect on efflux-pump gene expression in fluconazole-resistant Candida albicans clinical isolates. International Journal of Microbiology. 2020: 5989206.
Khalilzadeh, P., Lajoie, B., El Hage, S., Furiga, A., Baziard, G., Berge, M., et al. 2010. Growth inhibition of adherent Pseudomonas aeruginosa by an N-butanoyl-L-homoserine lactone analog. Canadian Journal of Microbiology. 56(4): 317-325.
Khiddee, J., Mongkolchaiarunya, S., Pochanukul, N., Jintakanon, P., Sukhumalin, P. 2018. The 8th National oral health survey report, 2017 (1 ed.). Bangkok: Sam Charoen Panich Ltd.Co.
Koseki, Y., Tanaka, R., Murata, H. 2018. Development of antibacterial denture cleaner for brushing containing tea tree and lemongrass essential oils. Dental Materials Journal. 37(4): 659-666.
Madeira, P. L., Carvalho, L. T., Paschoal, M. A., de Sousa, E. M., Moffa, E. B., da Silva, M. A., et al. 2016. In vitro effects of lemongrass extract on Candida albicans biofilms, human cells viability, and denture surface. Frontiers in Cellular and Infection Microbiology. 6: 71.
Naik, A. V. and Pai, R. C. 2011. A study of factors contributing to denture stomatitis in a north Indian community. International Journal of Dentistry. 2011: 589064.
Paranhos, H. F., Silva-Lovato, C. H., de Souza, R. F., Cruz, P. C., de Freitas-Pontes, K. M., Watanabe, E., et al. 2009. Effect of three methods for cleaning dentures on biofilms formed in vitro on acrylic resin. Journal of Prosthodontics. 18(5): 427-431.
Raungsawat, P., Itthidecharon, C., Wanachantararak, P. 2019. Influence of Melaleuca cajuputi powell and Cymbopogon citratus essential oil formulated in alcohol free mouthwash against Candida albicans Culture. Chiang Mai Dental Journal. 40(3): 125-134.
Satthanakul, P., Taweechaisupapong, S., Luengpailin, S., Khunkitti, W. 2019. The antifungal efficacy of essential oils in combination with chlorhexidine against Candida spp. Songklanakarin Journal of Science and Technology 41(1): 144-150.
Siriyod, W., Wanachantararak, P., Sastraruji, T., Chaijareenont, P., Chaiyana, W., Sookkhee, S., et al. 2023. Novel denture cleanser formulated from virgin coconut oil and the anionic emulsifier against Candida albicans biofilms formed on 96-wells plate and acrylic resin surfaces. Natural and Life Sciences Communications. 22(3): e2023047.
Sookkhee, S., Sakonwasun, C., Mungkornasawakul, P., Khamnoi, P., Wikan, N., Nimlamool, W. 2022. Synergistic effects of some methoxyflavones extracted from rhizome of Kaempferia parviflora combined with gentamicin against carbapenem-resistant strains of Klebsiella pneumoniae, Pseudomonas aeruginosa, and Acinetobacter baumannii. Plants (Basel). 11(22): 3128.
Taweechaisupapong, S., Aieamsaard, J., Chitropas, P., Khunkitti, W. 2012a. Inhibitory effect of lemongrass oil and its major constituents on Candida biofilm and germ tube formation. South African Journal of Botany. 81: 95-102.
Taweechaisupapong, S., Ngaonee, P., Patsuk, P., Pitiphat, W., Khunkitti, W. 2012b. Antibiofilm activity and post antifungal effect of lemongrass oil on clinical Candida dubliniensis isolate. South African Journal of Botany. 78: 37-43.
Wińska, K., Mączka, W., Łyczko, J., Grabarczyk, M., Czubaszek, A., Szumny, A. 2019. Essential oils as antimicrobial agents-myth or real alternative? Molecules. 24(11): 2130.
OPEN access freely available online
Natural and Life Sciences Communications
Chiang Mai University, Thailand. https://cmuj.cmu.ac.th
Rarinthorn Harintharanon1, Chintana Itthidecharon1, Phenphichar Wanachantararak2, and Siriwoot Sookkhee3, *
1 Department of Family and Community Dentistry, Chiang Mai University, Chiang Mai 50200, Thailand.
2 Dental Research Center, Faculty of Dentistry, Chiang Mai University, Chiang Mai 50200, Thailand.
3 Department of Microbiology, Faculty of Medicine, Chiang Mai University, Chiang Mai 50200, Thailand.
Corresponding author: Siriwoot Sookkhee E-mail: siriwoot.s@cmu.ac.th
Total Article Views
Editor: Pachara Sattayawat
Chiang Mai University, Thailand
Article history:
Received: January 17, 2023;
Revised: September 28, 2023;
Accepted: October 3, 2023;
Online First: October 10, 2023

