
Cleistocalyx nervosum var. paniala Extract: Anti-acne, Anti-oxidant, Anti-inflammatory Activities and Safety for Cosmeceutical Applications
Tharadon Khammitham, Pimporn Leelapornpisid, Sunee Chansakaow, Warat Leelapornpisid, and Worrapan Poomanee*Published Date : August 30, 2023
DOI : https://doi.org/10.12982/NLSC.2023.064
Journal Issues : Number 4, October-December 2023
Abstract This research aimed to investigate the biological activities and safety of extracts from Cleistocalyx nervosum var. paniala for anti-acne cosmeceutical and to determine total phenolic, flavonoid and anthocyanin contents and HPLC fingerprint. The extracts named as whole fruit extract (WF) and seed extract (SE), were macerated with 95% ethanol. Antibacterial effects against Cutibacterium acnes and Staphylococcus aureus were evaluated using agar diffusion, broth microdilution and time-kill kinetic assays. The results revealed that SE presented stronger antibacterial activities against C. acnes and S. aureus with inhibition zone of 22.74 ± 0.52 and 12.67 ± 0.15 mm, respectively. However, the MIC and MBC values were with 6.25 and 3.12 mg/ml for C. acnes and S. aureus, respectively, with a kill time of 2 and 4 h, respectively. SE showed higher phenolic and flavonoid contents compared with WF. In contrast, WF revealed higher anthocyanin content. According to HPLC analysis, ellagic acid was used as a marker. Additionally, anti-oxidant activity of the extracts was investigated by DPPH assay and inhibition on nitric oxide radicals. SE showed better activities with IC50 of 7.51 ± 0.40 and 25.47 ± 1.09 µg/ml, respectively. Therefore, SE was selected for testing with nitric oxide radical producing on RAW 264.7 cell lines which presented 22.99% inhibition. Moreover, SE showed no irritation on Hen's Egg Test Chorioallantoic Membrane. To conclude, SE from C. nervosum exerted stronger biological activities than WF. It also demonstrated a good potential concerning antibacterial on acne-related microorganisms, anti-oxidants, and anti-inflammatory activities. Therefore, it could be a promising bio-active compound to further develop into anti-acne cosmeceutical products.
Keywords: Cleistocalyx nervosum, Acne vulgaris, Cutibacterium acnes, Anti-inflammatory activity
Funding: The authors are thankful for the research funding supported by the Teaching Assistant and Research Assistant scholarships from the Graduate School and Faculty of Pharmacy, Chiang Mai University, Thailand.
Citation: Khammitham, T., Leelapornpisid, P., Chansakaow, S., Leelapornpisid, W., and Poomanee, W. 2023. Cleistocalyx nervosum var. paniala extract: Anti-acne, anti-oxidant, anti-inflammatory activities and safety for cosmeceutical applications. Natural and Life Sciences Communications. 22(4): e2023064.
INTRODUCTION
Acne vulgaris is one of the common skin diseases occurring within the pilosebaceous unit. Indeed, 77 to 89% of teenagers, with 82% of young men and 92% of young women in all ethnicity, confront physical and psychological problems from acne. The four fundamental mechanisms contributing to the development of acne are increased sebum production, follicular hyperkeratinization, skin bacterial colonization and inflammatory responses. Cutibacterium acnes, one of the resident skin flora, is considered as a major contributor involved in complex immune responses (Mawardi et al., 2021). In response to microbial compounds, C. acnes has the potential to initiate a series of inflammatory reactions in the lesion resulting in the neutrophil chemotaxis. Subsequently, neutrophils secrete a significant amount of reactive oxygen species (ROS) including nitric oxide contributing to the attenuation of the microorganisms with the disruption of follicular epithelium of the surrounding tissues and potentiate inflammation (Thielitz et al., 2001; Koreck et al., 2003; Dwyer et al., 2009). Also, other micro-organisms exacerbate acne inflammation, such as Staphylococcus aureus, Staphylococcus epidermidis, and Micrococcus spp. (Dhillon and Varshney, 2013). As a result, antibiotics have been employed to alleviate the number of bacteria on the skin and to ameliorate inflammation. Unfortunately, antibiotic-resistant bacteria easily occurred, eventually resulting in subsequent therapeutic failure (Arsa, 2008).
Consequently, a number of research studies have been conducted regarding new substances, drugs or medicinal plants to inhibit acne-causing bacteria. Currently, medicinal plants are increasingly employed to replace synthetic medications because they are generally considered safe materials and readily available, with a wide range of pharmacologic properties. Consequently, research exploring the anti-acne-inducing bacteria effects of medicinal plants potentially establish novel bio-active compounds to replace antibiotics in anti-acne cosmeceuticals (Arsa, 2008).
Cleistocalyx nervosum var. paniala, namely, Makiang, is a perennial plant grown widely throughout northern Thailand. C. nervosum, belonging to the Myrtaceae family, is consumed in the forms of juice, wine, nectar and jam. Several studies found that C. nervosum is a rich source of anthocyanins. The bright red fruit offers many health benefits, including lowering the risk of diabetes, cardiovascular disease, cancer, arthritis and neurological disorders. Moreover, C. nervosum fruit extracts contain bio-active compounds such as catechin, gallic acid, and rutin. Prasanth et al. (2020) reported that the leaf extract of C. nervosum contained high quantities of phenolic compounds, exerting a potential anti-oxidant effect. C. nervosum seeds, considered a waste product discarded from C. nervosum juice industries, showed anti-oxidant and cancer-preventive properties owing to its tannin. Furthermore, the SE is effective against C. acnes, a crucial acne-causing bacterium. However, industrially, separating seeds from whole fruit is costly, difficult and time-consuming. In addition, no evidence has been reported regarding the effect of C. nervosum whole fruit, including fruit and seeds, against acne-causing bacteria along with anti-inflammation, anti-oxidant, and safety profiles.
Therefore, this research aimed to evaluate and compare the biological activities of C. nervosum whole fruit and seed extracts in terms of anti-bacterial and anti-oxidant properties together with determining these extracts' phytochemical constituents. In addition, the anti-inflammatory effect, safety profiles, and physicochemical properties of the selected extracts were evaluated to further develop into anti-acne cosmeceuticals.
MATERIALS AND METHODS
Chemical materials
Thioglycollate agar, tryptic soy agar (TSA) and Tryptic soy broth (TSB) were purchased from Difco™ (Bangkok, Thailand). Thioglycollate broth was obtained from Merck (Bangkok, Thailand). Standard substances gallic acid (GA), quercetin (QU) and ellagic acid (EA) were purchased from Sigma-Aldridge (Schnelldorf, Germany). Triamcinolone acetonide (TA) was acquired from Sigma-Aldridge (Fluka Biochemika Company, Germany). Acetonitrile and methanol used as high-performance liquid chromatography (HPLC) eluents were HPLC-grade. All of the solvents and chemicals were of analytical grade.
Plant materials
From July to August 2021, C. nervosum fruits were purchased from Maejo-Phrae Campus, Phrae Province, Thailand. The fresh samples were separated into two parts; fresh seeds and whole fruit (fruits and seeds). They were frozen at -20 °C until use.
Preparation of C. nervosum extract
Two parts of fresh materials including the whole fruit and seeds of C. nervosum, were separately extracted using a maceration method. Briefly, the whole fruit was mashed together and extracted with 95% ethanol at a ratio of 1:2 (w/v) at room temperature for 24 h in three cycles. Likewise, the minced seeds were extracted using the same method as for the whole fruit. The filtrate from all cycles of each sample formerly filtered through Whatman No. 1 filter paper was pooled. A rotary evaporator (BuchiTM, RE120) was then employed to evaporate the ethanol from the filter solution. Eventually, concentrated WF and SE WF and SE were separately kept in tightly closed containers with light protection at 4°C before further investigation.
Determining phytochemical contents
Total phenolic content
The Folin-Ciocalteu (FC) assay was employed to determine the total phenolic contents of the extracts according to the method reported by Poomanee et al. (2018). In brief, 500.0 µl of each extract solution which was dissolved in 95% v/v of ethanol, was thoroughly mixed, then 2.0 ml of FC reagent and 4.0 ml of 7.5% w/v sodium carbonate (Na2CO3) was dissolved in de-ionized water. The mixture was then left at room temperature for 30 min. The absorbance of the reactive mixture at 765 nm was measured using an ultraviolet-visible (UV-Vis) spectrophotometer (Shimadzu® UV-2600i, Japan). The GA calibration curve was created presenting absorbance as a function of GA concentration. The following linear equation was employed to determine the total phenolic content of the extracts, expressed in terms of mg GA equivalent per gram of extract (mg GA/g extract).
Y = 7.2179X + 0.0087; R2 = 0.9991.
where Y is absorbance, and X is concentration of GA (mg/l).
Total flavonoid content
The total flavonoid content of the extracts was carried out using the method of Poomanee et al. (2015) with some modifications. Briefly, 1.0 ml of each extract solution, which was dissolved in 95% v/v of ethanol, was mixed with 0.3 ml of 5% sodium nitrite (NaNO2) and 4.0 ml of de-ionized water. The solution was left for 5 min before adding 0.3 ml of 10% aluminum chloride (AlCl3) for 3 min of incubation. Next, 2.0 ml of 1M sodium hydroxide (NaOH) was added to neutralize the reaction, and 2.4 ml of de-ionized water was added to adjust the volume to 10.0 ml. The mixture was then left at room temperature for 15 min, and the resulted absorbance at 510 nm was measured using a UV-VIS spectrophotometer. QU calibration curve presenting absorbance as a function of QU concentration was created. The following linear equation was used to determine the total flavonoid content of the extracts, expressed in terms of mg QU equivalent per gram of extract (mg QU/g extract).
Y = 0.6595X-0.0012; R2 = 0.9990.
where Y is absorbance, and X is concentration of QU (mg/l).
Total monomeric anthocyanin pigment content
The total monomeric anthocyanin pigment content of the extract was determined using the method of Lee et al. (2005) with modifications. In brief, 2.5 ml of each extract solution was transferred to a 25.0 ml volumetric flask with 0.025 M potassium chloride (KCl) buffer, pH 1.0. Then the sample solution was transferred in a volumetric flask with 0.4 M sodium acetate (CH3CO2Na) buffer, pH 4.5, and incubated for 15 min. The absorbance of the mixture at 520 and 700 nm were measured using a UV-Vis spectrophotometer. The monomeric anthocyanin pigment content was calculated using the equation in cyanidin-3-glucoside equivalents below.
Anthocyanin pigment (mg/l) = (A×Mw×DF×1000)
(Ɛ×1)
Where A is absorbance, Mw is the molecular weight of cyanidin-3-glucoside (CNG) (449.2 g/mol), DF is a dilution factor, Ɛ is 26,900 molar extinctive coefficients in L/mol-1cm-1, and 1 is pathlength in cm.
Antimicrobial activity
Bacterial strains
The antimicrobial protocol of this research was approved for implementation by the Institutional Biosafety Committee of Chiang Mai University, Thailand (CMUIBC A-0565001). The standard bacterial suspension of C. acnes (DMST 14916) and S. aureus (DMST 8840) were supplied from the Department of Medical Sciences, Ministry of Public Health, Thailand.
Bacterial suspensions
The bacterial suspensions of C. acnes and S. aureus were cultivated in modified thioglycollate broth pH 7.4 and TSB, respectively. Then C. acnes was inoculated onto modified thioglycollate agar pH 7.4 and incubated in an anaerobic jar with an anaerobic gas pack (AnaeroGenTM, Oxoid Ltd.) at 37 ± 2 °C for 72 h. Then, two to three colonies of C. acnes were inoculated in the thioglycollate broth and incubated under anaerobic conditions at 37 ± 2 °C for 72 h before determining antimicrobial effect. In the case of S. aureus, the bacterial suspension was inoculated onto TSA and incubated at 37 ± 2 °C for 24 h. Also, two to three colonies of S. aureus were then inoculated into TSB and incubated at 37 ± 2°C for 24 h before testing (Poomanee et al., 2018). The suspension of C. acnes and S. aureus were diluted using the thioglycollate broth and TSB to produce final concentrations of approximately 1×108 and 1×106 CFU/ml with an optical density (OD600) of 0.1, respectively.
Agar disc diffusion
The antimicrobial activities of the C. nervosum extracts were firstly determined using the agar disc diffusion assay, modified from Poomanee et al. (2018). The diluted suspension of C. acnes in the thioglycollate broth and S. aureus in TSB were applied evenly onto the thioglycollate agar and TSA, respectively, then a sterile paper disc (MACHEREY-NAGEL GmbH Co., Germany) 6 mm diameter was soaked into each concentration of sample solution dissolved in 10% DMSO in phosphate-buffered saline (PBS) pH 7.4. The sterile paper disc was then plated onto the agar of each of the bacteria strains. The petri dish containing C. acnes was incubated at 37 ± 2 °C for 72 h under an anaerobic conditions in an anaerobic jar. On the other hand, the petri dish containing S. aureus was incubated at 37 ± 2 °C for 24 h under aerobic conditions. Clindamycin; CM (10 mg/l), erythromycin; ERY (10 mg/l), GA (100 mg/ml), QU (100 mg/ml) and EA (100 mg/ml) were used as positive controls, while the negative control was 10% DMSO in PBS. Mean diameter of inhibition zone was measured in mm, including the 6 mm diameter of the paper disc.
The broth dilution method
The broth dilution method determining the minimum inhibitory concentrations (MIC) and minimal bactericidal concentration (MBC) was carried out following the method of Wayne, (2020). Briefly, each sample solution (50 µl) was two fold serially diluted by 10% DMSO in PBS in U-shape 96-well plates. The bacterial suspension (50 µl), was adjusted to an OD600 0.10, then added to each well. The plates of C. acnes were incubated at 37 ± 2 °C for 72 h under anaerobic conditions, and for S. aureus, the plates were incubated at 37 ± 2 °C for 24 h. The MIC of each sample, defined as the lowest concentration of extracts inhibiting the growth of bacteria, was investigated. CM (100 mg/l), ERY (100 mg/ml), GA (100 mg/ml), QU (100 mg/ml) and EA (100 mg/ml) served as positive controls, and the negative control was 10% DMSO in PBS.
In addition, MBC was defined as the lowest concentration with no visible bacterial strain growth; the well with no growth of bacteria was collected and dropped onto thioglycollate agar for C. acnes and TSA for S. aureus. The agar plates were then incubated under appropriate conditions for different bacterial strains.
Time-kill kinetics
The Time-kill kinetic assay was performed according to Poomanee et al. (2018) with modifications. Briefly, the suspension of bacteria OD600 0.10 was added to each extract solution at a ratio of 1:1. After that, 130.0 µl of aliquot was transferred to 7% w/v polysorbate 80 (1,170.0 µl) in TSB, functioning as a neutralizing solution inactivating polyphenol (Rowe et al., 2009) which was collected at 0, 2, 4 and 8 h for C. acnes and 0, 1, 2, 4 and 8 h for S. aureus. The spreading plate method was used to count colony-forming units (CFU). Next, plates were incubated at 37 ± 2 °C for 72 h under anaerobic conditions for C. acnes and incubated at 37 ± 2 °C for 24 h under aerobic conditions for S. aureus. GA was used as a positive control (data not shown). Moreover, bacterial cultures were used as solvent control. Each extract's time-kill curve was calculated and reported as time and log10 survivor curves.
Anti-oxidant activity
2, 2-Diphenyl-2-picrylhydrazyl (DPPH) radical scavenging assay
The DPPH radical scavenging assay was performed according to the modified method of Maneechai et al. (2023). Each extract was dissolved in 95% ethanol to make serial concentrations (6.25 to 10.0 µg/ml). Each extract solution (20.0 µl) was mixed with 180 µl of 120 mM DPPH ethanolic solution and incubated for one half hour in the dark. The ethanol (20.0 µl) was mixed with DPPH 180.0 µl, and used as a negative control. Ethanol (200.0 µl) was used as a solvent control. GA, QU and EA were used as positive controls. The absorbance of the individual extract was measured at 520 nm using a microplate reader (SpectraMax M3, USA). The equation below was used to compute the percentage of inhibition.
DPPH scavenging activity (%) = (Bc-Bs) ×100.
Bc
where Bc is the absorbance of the negative control, and Bs is the absorbance of the sample. The 50% inhibitory concentration (IC50) was calculated using the graph of individual extracts plotted between percent inhibitions (Y) and concentrations (X).
Nitric oxide radical scavenging assay
The nitric oxide radical scavenging assay was performed with modifications, according to Srihaphon et al. (2020). Briefly, 60.0 µl of sodium nitroprusside (SNP) aqueous solution (10 mM) was thoroughly mixed with 20.0 µl of PBS pH 7.4 and 20.0 µl of C. nervosum extract in various concentrations (0.625 to 10.0 mg/ml). The mixture was incubated for 150 min at room temperature. After that, 50.0 μL of 1% sulfanilamide in 2% phosphoric acid (H3PO4) was added and incubated for 5 min at room temperature. Next, 50.0 μL of 0.1% N-(1-Naphthyl) ethylenediamine dihydrochloride (NED) was added to the solution, and then incubated for 10 min at room temperature. GA, QU and EA were used as positive controls. The absorbance of the pink mixture at 540 nm was measured using a microplate reader (SpectraMax M3, USA). The equation below was used to calculate the percentage of nitric oxide radical scavenging activity.
Nitric oxide scavenging activity (%) = (Bc-Bs) ×100.
Bc
where Bc is the absorbance of the control (reagents without the sample), and Bs is the absorbance of the sample (C. nervosum extract). The 50% inhibitory concentration (IC50) was calculated using the graph of individual extracts plotted between percent inhibitions (Y) and concentrations (X).
Anti-inflammatory activity
The extract showing high phytochemical contents and the best anti-bacterial and anti-oxidant properties was selected for studying the anti-inflammatory activity.
The inhibition of nitric oxide production from RAW 264.7 macrophage cell line by the Griess reagent was determined, as described by Torres-Rodríguez et al. (2016). The RAW 264.7 cell line was cultured in Dulbecco's Modified Eagle Medium containing 1% v/v PBS, penicillin (100 U/ml) and streptomycin (100 mg/ml) and seeded at a density of 2×105 cells on a 6-well plate at 37°C for 48 h in a 5% CO2 atmosphere. Then the cells were induced with LPS and treated with SE sterilized through a pore membrane (0.2 μm) in serial concentrations ranging from 0.0010 to 0.10 mg/ml or TA for 24 h. Then the cell culture medium was transferred to a 96-well plate, mixed with an equal volume of Griess reagent (1% w/w sulfanilamide in 2% w/w H3PO4 and 0.1% NED), and incubated at room temperature for 5 min. The absorbance was measured at 540 nm using an Ultra Microplate Reader (Biotek, USA). The presence of nitrite oxide was determined, and the results were expressed as a percentage of nitric oxide reduction using the equation below.
Nitric oxide reduction (%) = (Bc-Bs) ×100.
Bc
Where Bc is the absorbance of cells treated with medium containing LPS and Bs is the absorbance of cells treated with the extract and LPS.
HPLC analysis
Since SE showed high phytochemical contents with the best anti-bacterial and anti-oxidant properties, it was thus selected for phytochemical analysis by HPLC. The phytochemical constituents of the selected extract were further identified and quantified using HPLC. The assay conditions were performed on a KINETEX® C18 (LC column 150 × 4.6 mm, 5 μm) and analyzed using Shimadzu HPLC (Shimadzu, Japan).
The mobile phase was composed of solvent A: 0.1% formic acid in de-ionized water at pH 2.70 and solvent B: acetonitrile. The gradient elution profiles were set up following the modified method of Poontawee et al. (2016) with modifications. The initial mobile phase consisted of 0-5 min: 100% A; 5 to 10 min: 95% A; 10 to 20 min: 90% A; 20 to 30 min: 85% A: 30 to 35 min: 80% A; 35 to 40 min: 60% A and 40 to 50 min: 100% A. The operating temperature was kept constant at 40oC throughout the analysis, with a flow rate of 1 ml/min. The phenolic components in the SE were identified and quantified by comparing the peaks of UV spectra at a retention time of 270 nm to authentic standards. The identified compounds were quantified by comparing their respective standards' peak area calibration curves, which are EA. In addition, the HPLC method validation of the ellagic acid standard was also executed.
Irritation test: Hen's egg test chorioallantoic membrane (HET-CAM) assay
The irritation investigation used a modified hen's egg test chorioallantoic membrane (HET-CAM) assay performed according to the method of Chaiyana et al. (2017). Briefly, eggshell tissue (chorio-allantoic-membrane) was prepared from fertilized chicken eggs for 7 to 9 days. Air pockets were cut off using a rotating dentist saw blade and the egg was gently peeled. Then a drop of PBS pH 7.0 solution was added, and the eggs were incubated for 15 min. A forcap was used to remove the inner eggshell membrane, 30 μl of test reagent was added, and the blood vessel network was observed short term for 5 min and long term for 60 min. The images of the CAM were then recorded using a Lumix digital camera under a microscope (Panasonic, Beijing, China). The positive control was 1% w/v sodium lauryl sulfate (SLS), and the negative control and vehicle control were 0.9% w/v sodium chloride (NaCl). The time was observed to note the lack of vascular hemorrhage, lysis, and coagulation and the irritation score (IS) was determined using the equation below.
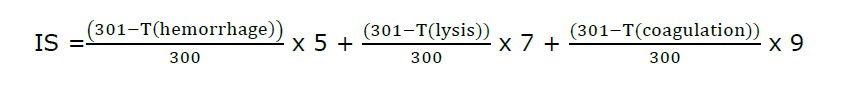
where T(hemorrhage) is the time of the first vascular hemorrhage, T(lysis) is the time of the first vascular lysis, and T(coagulation) is the time of the first vascular coagulation. The irritation score (IS) was then calculated as follows: 0.0 to 0.9 equals no irritation; 1.0 to 4.9 equals mild irritation; 5.0 to 8.9 equals moderate irritation; and 9.0 to 21.0 equals severe irritation (Freire et al., 2015).
Statistical analysis
All data were carried out in triplicate, and results were expressed as mean ± SD. The samples' antibacterial, anti-oxidant, and anti-inflammation properties were compared using statistical analysis by One-Way ANOVA and Tukey multiple comparison tests through SPSS Software, Version 26.0, with a P < 0.05 that was considered statistically significant.
•RESULTS
Plant extraction
Table 1 presents that the SE showed a higher percent yield than the WF. The characteristics of WF and SE were obviously differed. WF was a reddish-black appearance, viscous semisolid and had a distinctive smell, while SE was a sticky texture, brown to black with a distinct odor, and had higher viscosity compared with WF.
Table 1. Percentage yields and characteristics of C. nervosum extracts.
|
Extract |
Fresh weight of Plant (g) |
Weight of crude extract (g) |
Extract yield (%w/w) |
Characteristic |
|
Whole fruit extract |
500.73 |
18.41 |
3.67% |
Black-red, viscous semisolid |
|
Seed extract |
600.37 |
26.90 |
4.48% |
Dark brown, viscous semisolid |
Phytochemical contents
As shown in Figure 1, the results revealed that the total phenolic contents of WF and SE were 212.25 ± 0.19 and 386.87 ± 0.98 mg of GA/g of extract, respectively, (Figure 1). Moreover, the total flavonoid contents of WF and SE were 76.22 ± 0.09 and 117.41 ± 0.23 mg of QU/g of extract, respectively. Additionally, the total anthocyanin contents of WF and SE were 226.55 ± 6.32 and 123.02 ± 5.86 mg of CNG/100 g extract, respectively. Interestingly, SE had significantly (P <0.05) higher phenolic and flavonoid contents than WF. In contrast, WF contained significantly (P <0.05) higher amounts of anthocyanin content than SE.
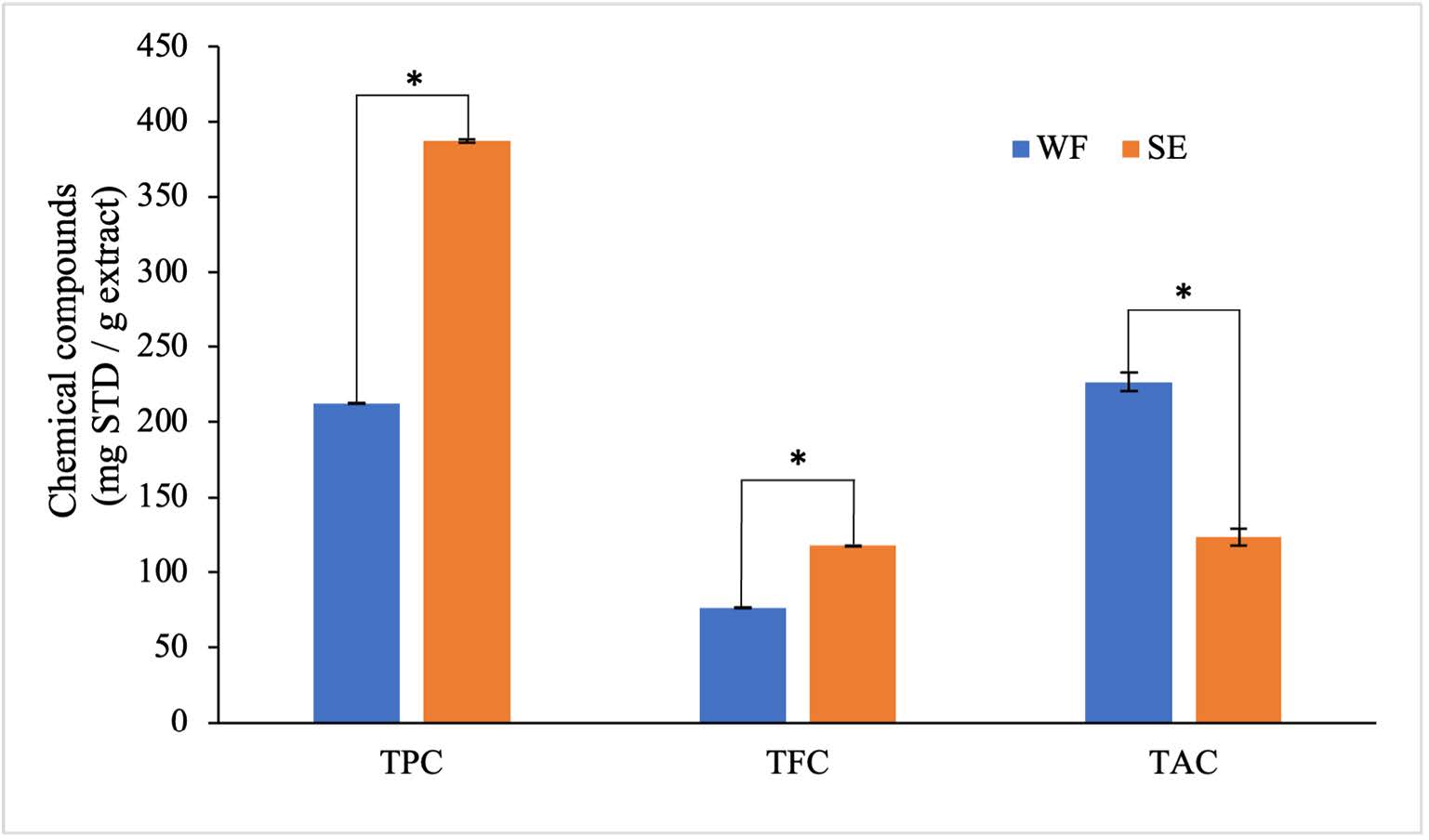
Figure 1. Total phenolic, flavonoid and anthocyanin contents of C. nervosum extracts
Note: Each value was expressed as mean ± SD (n=3) of three independent experiments; (*) values presented a significant difference (P <0.05) analyzed using the independent samples t-test. STD: Standard, WF: Whole fruit extract, SE: Seed extract, TPC: Total phenolic content, TFC: Total flavonoid content, TAC: Total anthocyanin content.
Antibacterial activities
The results of the anti-bacterial activity by the agar disc diffusion method as shown in Table 2 found that CM and ERY at 10 mg/L had significantly higher inhibitory effects against all tested microorganisms than the extracts. Interestingly, SE showed a higher inhibition zone against C. acnes than WF, GA, QU and EA which exhibited significant differences (P <0.05). Moreover, the effect of SE against S. aureus significantly differed from that of WF, GA, QU and EA. However, inhibition against C. acnes of the extracts was greater than inhibition against S. aureus.
Table 2. Diameter of inhibition zone in mm against C. acnes and S. aureus of WF, SE and standard substrates.
|
Sample/Standard |
Diameter of inhibition zone (mm) |
|
|
C. acnes |
S. aureus |
|
|
WF |
13.78 ± 0.22e |
10.36 ± 0.31c |
|
SE |
22.74 ± 0.52c |
12.67 ± 0.15b |
|
GA |
7.02 ± 0.46f |
7.21 ± 0.89d |
|
QU |
NI |
NI |
|
EA |
16.50 ± 0.23d |
14.50 ± 0.21b |
|
CM* |
40.50 ± 0.31a |
21.59 ± 0.33a |
|
ERY* |
30.48 ± 0.16b |
12.07 ± 0.61b |
Note: Paper disc with 6 mm diameter, NI: No Inhibition, results expressed as mean ± SD of three independent experiments. Superscript letters (a, b, c, d, e and f) presented in different letters within the same column differ significantly (P <0.05) by analyzing the One-Way ANOVA with multiple comparisons using Tukey’s method. Concentrations of a samples, GA, QU, and EA at 100 mg/ml. (*) represent the concentration of CM and ERY at 10 mg/l. WF: Whole fruit extract, SE: Seed extract, GA: Gallic acid, QU: Quercetin, EA: Ellagic acid, CM: Clindamycin, ERY: Erythromycin.
Aside from inhibition zone, the MIC and MBC values of the tested samples are shown in Table 3. The results demonstrate that the bactericidal effects against C. acnes and S. aureus of CM and ERY which are antibiotics used to treat topical acne were outstanding among all samples owing to the lowest MIC and MBC values. Interestingly, SE showed a remarkable bactericidal effect against C. acnes and S. aureus compared with that of WF, GA, EA and QU. However, no MBC value against C. acnes and S. aureus of quercetin was shown which was inconsistent with the results from agar disc diffusion. Noticeably, antimicrobial activity of SE was superior to those of phenolic-positive controls.
Table 3. The MIC and MBC of WF and SE from C. nervosum against C. acnes and S. aureus.
|
Sample/Standard |
Susceptibility of bacteria (mg/ml) |
|||
|
C. acnes |
S. aureus |
|||
|
MIC |
MBC |
MIC |
MBC |
|
|
WF |
12.50 |
12.50 |
6.25 |
6.25 |
|
SE |
6.25 |
6.25 |
3.12 |
3.12 |
|
GA |
12.50 |
12.50 |
50.00 |
50.00 |
|
QU |
100.00 |
NI |
100.00 |
NI |
|
EA |
3.12 |
6.25 |
2.50 |
5.00 |
|
CM |
0.49* |
0.49* |
3.91* |
3.91* |
|
ERY |
48.83* |
48.83* |
97.66* |
97.66* |
Note: NI: No Inhibition, WF: Whole fruit extract, SE: Seed extract, GA: Gallic acid, QU: Quercetin, EA: Ellagic acid, CM: Clindamycin, ERY: Erythromycin, MIC: minimum inhibitory concentration, MBC: minimum bactericidal concentration.
*MIC and MBC values of CM and ERY were expressed in mg/l.
The WF and SE in concentrations of MBC, 2MBC and 3MBC, respectively, were then determined for time-kill kinetics expressed as time and function of log10 survivors as shown in Figure 2. The viable counts of C. acnes and S. aureus which were treated with WF and SE were decreased in a dose-dependent manner. Generally, a 3-log10 reduction in log CFU/ml is considered as a bactericidal effect (Keepers et al., 2014). The result showed that the WF at MBC exerted a 4-log10 reduction against C. acnes after 2 h of incubation. However, regrowth occurred after 4 h. In the case of SE at MBC, a 3-log10 reduction was observed after 2 h of exposure. However, regrowth was found after 4 h. At 2MBC and 3MBC, the WF and SE reached about a 5-log10 reduction after 2 h of incubation. However, at 2MBC and 3MBC of all samples, regrowth still occurred after 4 h (Figure 2).
Furthermore, the effect against S. aureus of the WF at MBC demonstrated a 4-log10 reduction after 4 h of exposure with no regrowth. In addition, the SE at MBC, exerted a 5-log10 reduction after 4 h of incubation. At 2MBC and 3MBC, the WF reached about a 7-log10 reduction after 4 h of exposure with no regrowth. Moreover, 2MBC and 3MBC of SE exerted a 5-log10 reduction after 4 h of incubation. However, regrowth occurred after 4 h after exposure to the SE at 2MBC and 3MBC, as shown in Figure 2.
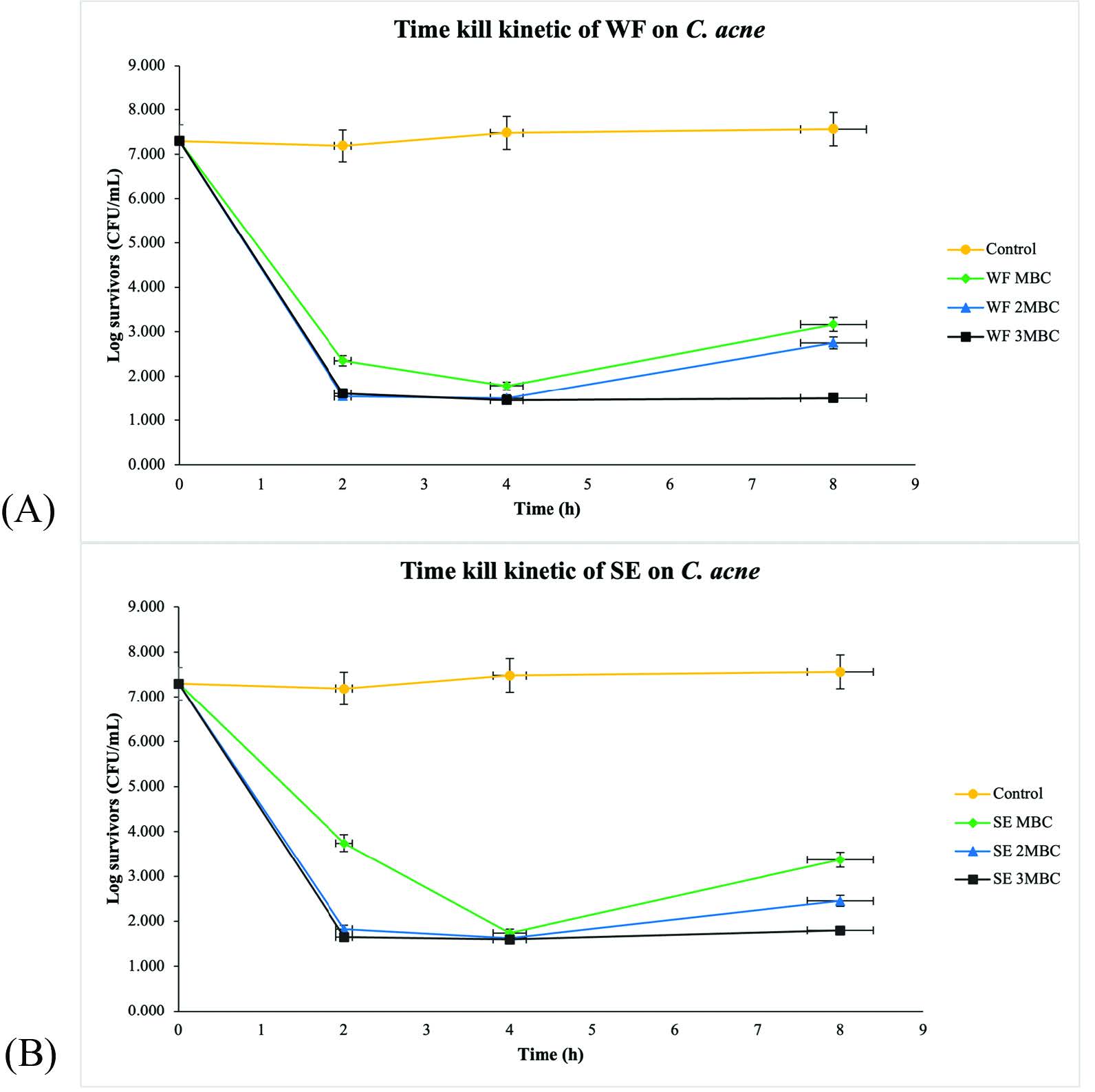
Figure 2. Time-kill curves of (A) WF and (B) SE against C. acnes, (⯁) 1MBC, (▲) 2MBC, (■) 3MBC; (⚫) Negative control. WF: Whole fruit extract, SE: Seed extract
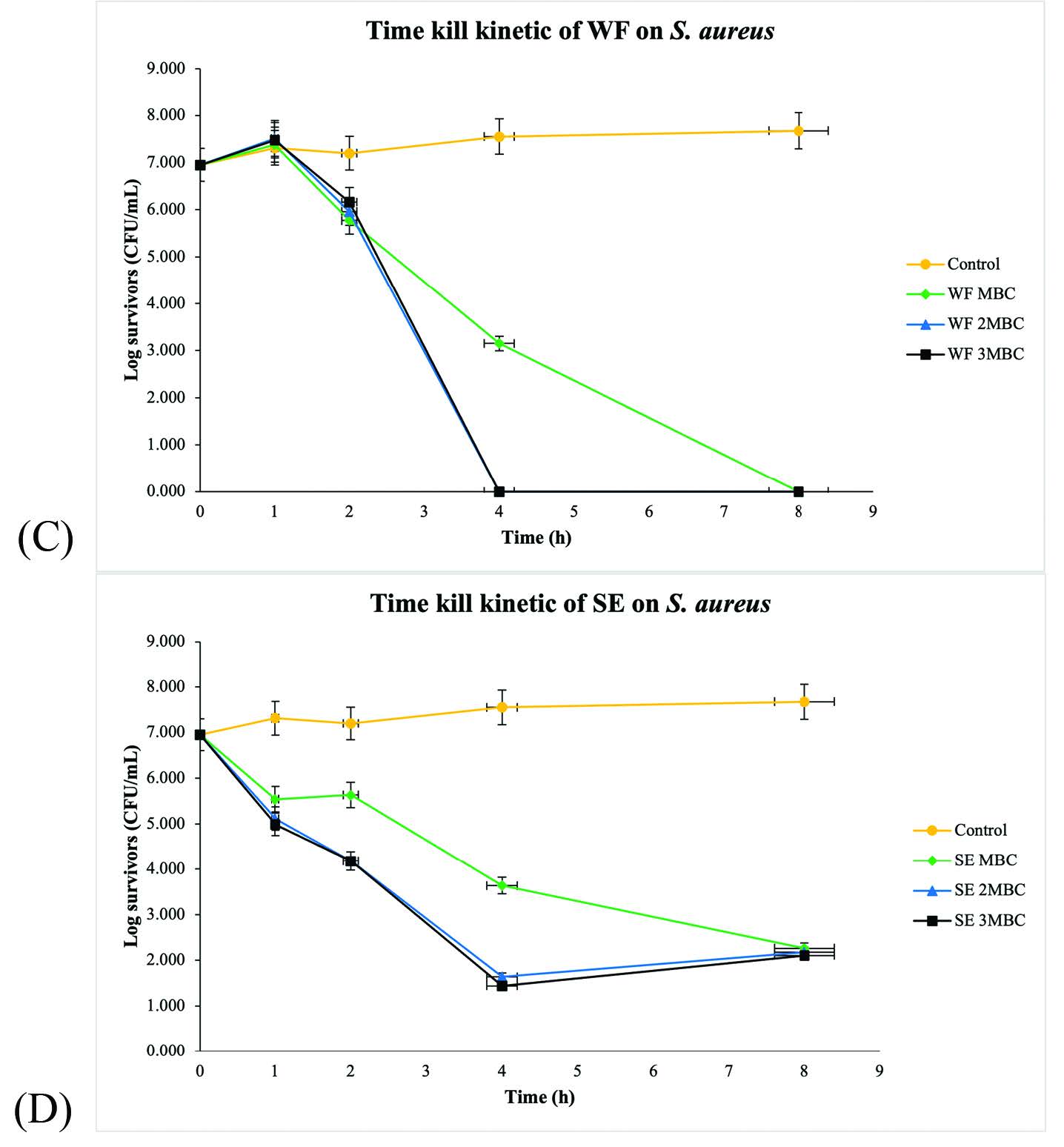
Figure 2. Time-kill curves of (C) WF and (D) SE against S. aureus, (⯁) 1MBC, (▲) 2MBC, (■) 3MBC; (⚫) Negative control. WF: Whole fruit extract, SE: Seed extract.
Anti-oxidant activity
Table 4 illustrates that all the standards including GA, QU and EA exhibited strong inhibitory effect on DPPH and nitric oxide radicals with lower IC50 values than extracts. Interestingly, the anti-oxidant effect of SE was superior to that of WF, which corresponded to their total phenolic and flavonoid contents. Moreover, SE showed stronger nitric oxide radical scavenging activity than EA.
Table 4. IC50 values (µg/ml) of anti-oxidant activity using the DPPH and nitric oxide radical scavenging.
|
Sample/Standard |
Anti-oxidant activities (Mean ± SD.) (µg/ml) |
|
|
IC50 on DPPH |
IC50 on Nitric oxide |
|
|
WF |
13.52 ± 0.95c |
108.70 ± 3.31d |
|
SE |
7.51 ± 0.40b |
25.47 ± 1.09b |
|
GA |
1.18 ± 0.090a |
6.76 ± 0.23a |
|
QU |
1.49 ± 0.079a |
7.84 ± 0.59a |
|
EA |
1.69 ± 0.073a |
35.77 ± 0.58c |
Note: Results were expressed as mean ± SD of three independent experiments. WF: Whole fruit extract, SE: Seed extract, GA: Gallic acid, QU: Quercetin, EA: Ellagic acid, IC50: 50% inhibitory concentration. Superscript letters (a, b, c and d) presented in different letters within the same column differ significantly (P <0.05) by analyzing the One-Way ANOVA with multiple Tukey comparisons.
Anti-inflammatory activity
As aforementioned, the biological effects of SE in terms of anti-oxidant and anti-bacterial effects were superior to those of WF. Therefore, SE was selected for further investigation regarding the anti-inflammatory activity study. Figure 3 reveals that the SE showed an anti-inflammatory activity on LPS-induced RAW 264.7. At a concentration of 0.1 mg/ml of SE and TA, they potentially inhibited the release of nitric oxide from LPS-induced RAW 264.7 using maximum percent inhibition of 22.99 ± 2.54% and 27.88 ± 4.74%, respectively. Moreover, the inhibitory effects on nitric oxide production of SE and TA were dose-dependent. Additionally, the SE presented significant difference from TA at all doses (P <0.05).

Figure 3. The % inhibition of nitric oxide (NO) production of SE and TA on LPS-induced RAW 264.7 cell line; (Mean ± SD; n = 4). SE: Seed extract, TA: Triamcinolone acetonide.
HPLC analysis
Figure 4 illustrated that SE contained EA at the retention time of 33.244, and the standard curve of EA was consequently constructed using the linear equation of Y = 24667X - 18378, R² = 0.9994, where Y is the area under the curve (mAU) and X is concentrations of EA (ppm). According to the HPLC method validation, the limit of detection (LOD) and limit of quantitation (LOQ) of this separating condition were 0.4 and 2 ppm, respectively. In addition, accuracy and precision expressed as % recovery and % relative standard deviation (RSD) were more than 90% and less than 2%, which were 96.78% for accuracy and 1.00% for precision, respectively. (ICH, 2022)

Figure 4. HPLC chromatograms of (A) SE and (B) EA (100 ppm). SE: Seed extract, EA: Ellagic acid.
Irritation test: Hen's egg test chorioallantoic membrane (HET-CAM) assay
The HET-CAM assay was one of the most famous and convenient irritation research studies because ethics approval was not required when the age of the animal's embryo was less than one half of the overall incubation period.
Table 5 reveals the irritation score from the HET-CAM assay of SE together with positive and vehicle controls. No irritation induced by SE at 2.5% w/v solution was observed which was the 2MBC concentration of the SE similar to the vehicle control which was 0.9% w/v NaCl. In the case of 1% w/v SLS serving as a positive control, severe irritation was observed which is clearly illustrated in Figure 5. The blood vessels before and after the sample applications were employed for irritation detection. Vessel lysis could be produced with as little as 1% w/v SLS. On the contrary, after 60 min of exposure, no hemorrhage, lysis, and coagulation was detected in the vessel exposed to 2.5% w/v of SE and 0.9% w/v NaCl solution. As a result, SE at
2.5 % w/v was safe and caused no skin irritation.
Table 5. IS score and irritation level form HET-CAM assay (n = 3)
|
Sample |
Irritation score (IS.) |
Irritation Level |
|
SE (2.5 % w/v) solution in NaCl |
0.0 ± 0.0 |
No irritation |
|
Negative control (0.9% w/v NaCl) |
0.0 ± 0.0 |
No irritation |
|
Positive control (1% w/v SLS) |
12.8 ± 0.2 |
Severe irritation |
Note: SE: Seed extract, NaCl: Sodium chloride, SLS: Sodium lauryl sulfate.
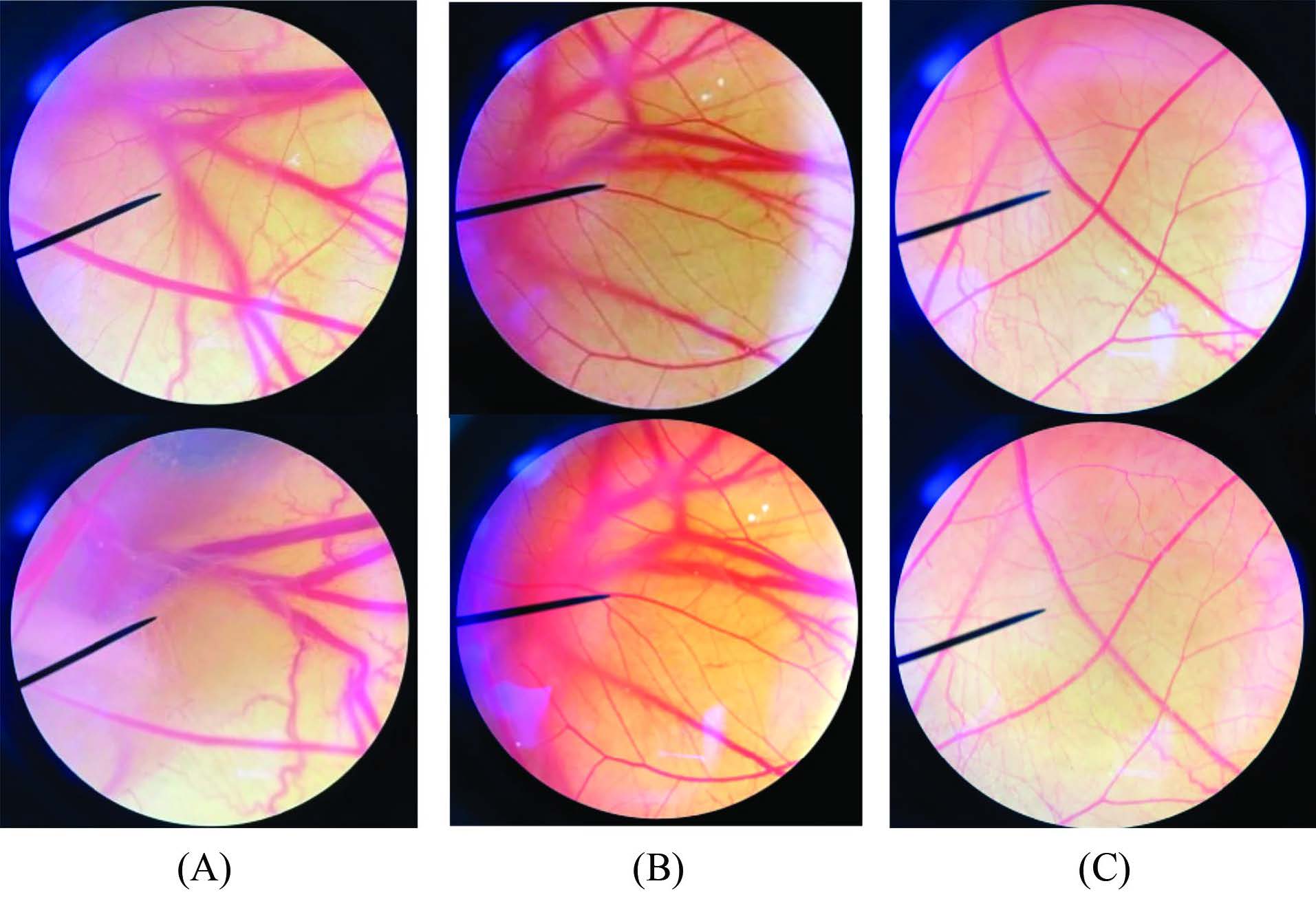
Figure 5. Photographs illustrating the effects of (A) 1% w/v SLS; (B) 0.9% w/v NaCl; and (C) SE (2.5 % w/v) solution in NaCl in HET-CAM assay before applying the sample (upper) and at the end of the test of 60 min (lower). SLS: Sodium lauryl sulfate, NaCl: Sodium chloride, SE: Seed extract.
DISCUSSION
In the present study, C. nervosum in form of SE, a waste product from fruit consumption, and WF were studied for their biological potential for anti-acne cosmetic products. In addition, WF was firstly investigated for its antibacterial and anti-oxidant properties together with its phytochemical constituents. Furthermore, the anti-inflammatory effect, safety profile and physicochemical properties of the extract were evaluated to establish its potential in the anti-acne cosmeceuticals. The characteristics of the obtained SE in our study was similar to SE from the study of Arsa (2008) in which sticky brownish SE was reported. This characteristic was expected to contain rubber or resin compounds, making extracted substances solidify more as the solvent evaporates. In the case of WF, the black red appearance of the extract might have resulted from the peel and pulp of C. nervosum indicating the presence of anthocyanins (Chariyakornkul et al., 2022).
Several factors significantly influenced the extraction of plants including time, solvent, temperature etc. (Zhang et al., 2018; Maneechai et al., 2023). This study evaluated the extraction condition which had an influence on phytochemical content and anti-oxidant activity. As a result, the total phenolic contents of SE and WF extracted within 24 h were higher than that of the ethanolic the seed extract reported in the related study of Maisuthisakul et al. (2007), presenting 173.6 ± 1.9 mg of GA/g of dry weigh of the 95% ethanol crude extract for 4.5 h. This finding might be resulted from the longer extraction time conducted in our study. Aside from extraction time, the polarity of the extraction solvents plays an important role in extracting efficacy for polyphenol extraction. One related study found that the pulp extract of C. nervosum extracted using distilled water contained total anthocyanin content of 50.49 ± 0.64 mg CNG/100 g dry weight and total phenolic content of 5.36 ± 0.09 mg GA/g dry weight (Sukprasansap et al., 2017) which were lower than our results. Generally, polar solvents like water, ethanol and methanol are preferable to extract highly hydroxylated aglycone forms of phenolic compounds (Kaczorová et al., 2021). The fruit and seed extracts of C. nervosum were reported to have GA, catechin, tannin and rutin as major phytochemical compounds (Poontawee et al., 2016) which are easily extracted by polar organic solvents such as ethanol and methanol rather than water corresponding to our results.
Polyphenols, secondary metabolites found in plants, chiefly contribute to potential human health benefits, especially as antibacterial, anti-oxidants, and anti-inflammation bio-active compounds (Daglia, 2012; Likittrakulwong et al., 2023). Our study revealed that SE and WF of C. nervosum, containing an outstanding amount of polyphenols, exerted notable antibacterial properties. According to the study of Bouarab-Chibane et al. (2019), the capacity of hydrogen binding of phenolic compounds to bacterial enzymes changed the permeability of cell membranes of micro-organisms, various intracellular processes and the rigidity of cell walls with integrity losses resulting in cell death. Interestingly, Bouarab-Chibane et al. (2019) reported that flavonoids may affect the synthesis of proteins and RNA by reducing energy and DNA synthesis of the bacteria. Moreover, flavonoids also interfere with the mechanisms producing bacterial energy (Adenosine triphosphate: ATP) and intracellular pH changes in gram-positive bacteria. In addition, Lertsatitthanakorn et al. (2010) found that phenolic compounds can disrupt bacterial cell membranes and cell walls, which can kill the bacteria. Therefore, altering cell membrane permeability and disrupting the cell membrane could be potential mechanisms of action against C. acnes of SE. According to a study by Sivasankar et al. (2016), a combination of EA and tetracycline effectively inhibited biofilm formation by C. acnes (80-91%) without affecting its growth. This finding suggests that EA possibly attenuated the biofilm of C. acnes, leading to the death of the bacteria. The MIC and MBC values against C. acnes and S. aureus of GA and CM, which served as a positive control in this study, were in a similar range to those in the study of Poomanee et al. (2018). The regrowth of bacteria found in our study according to time-kill kinetic curve could be interpreted using the hypothesis of persisters. Wood et al. (2013) reported that persisters are bacterial cells that can resist antibiotic treatment without genetic alteration presented in bacterial resistance. Persister cells do not multiply in the presence of antibiotics, in contrast to resistant cells that do. The presence of C. acnes and S. aureus after 2 and 4 h of incubations with SE, respectively indicated that persister cells resisting the polyphenols of the SE. However, no S. aureus persister cell were observed after incubating with WF.
During the acne inflammatory process, neutrophils are stimulated to produce an excessive number of ROS owing to the effect of interleukin (IL)-8 resulting from C. acnes immunomodulatory property. Eventually, ROS could cause bacterial degradation, tissue damage and oxidative stress exacerbating inflammatory responses (Sahu et al., 2015; Kardeh et al., 2019). Therefore, anti-oxidant compounds potentially suppress acne inflammation (Poomanee et al., 2018). Our results demonstrated that SE and WF presented potent inhibitory effects on free radicals in the forms of synthetic DPPH radical and nitric oxide providing an ameliorating effect on acne inflammation. Tsao, (2010) found that polyphenols were potent anti-oxidants capable of neutralizing free radicals by donating an electron or a hydrogen atom.
Furthermore, polyphenols prevent the creation of free radicals, slowing the oxidation rate by deactivating the active species and precursors of free radicals. Additionally, related research has shown that anti-oxidation potency is highly related to total phenolic content and flavonoid compounds in the plant extracts (Mustafa et al., 2010), corresponding to our results in which the anti-oxidant effect of SE was better than that of WF due to its higher total phenolic and flavonoids contents. Additionally, the mechanism of EA is a molecule that can scavenge free radicals by donating a hydrogen atom from its phenolic group. This process can occur through three mechanisms: hydrogen atom transfer, single electron transfer followed by proton transfer, and sequential proton loss electron transfer (Marković et al. 2013).
Nitric oxide is a signaling molecule playing an important role in the pathophysiology of acne inflammation (Sharma et al., 2007). Grange et al. (2009) found that CD36 and toll-like receptors (TLR)-2 were triggered by C. acnes resulting in forming oxygen radicals through the NADPH oxidase pathway (NOX) and interacting with nitric oxide molecules to generate peroxynitrite. The resulting peroxynitrite then activates p38 and ERK MAPKs eventually generating IL-8 as a neutrophil chemoattractant causing acne inflammation. Accordingly, inhibiting nitric oxide production potentially attenuates the acne inflammatory response (Qin et al., 2015). Herein, LPS was used as a mediator for RAW 264.7 a mouse macrophage cell line inflammation model because LPS is able to activate macrophages and monocytes to produce a variety of cytokines, such as tumor necrosis factor (TNF), IL-1 and IL-6, as well as inflammatory mediators including nitric oxide and other free radicals (Yang et al., 2012). Our study found that SE could inhibit nitric oxide synthesis and release from LPS-activated RAW 264.7 cells implying the anti-inflammatory property of the extract. Thus, LPS-induced inhibition of nitric oxide production, which leads to inflammation in macrophages, may be a promising mechanism of action inhibiting nitric oxide of SE. Polyphenols exert anti-inflammatory effects, particularly through their capacity to scavenge free radicals, control the cellular activities of inflammatory cells and regulate the activity of the enzymes involved in the metabolism of arachidonic acid (phospholipase A2, COX), arginine and other pro-inflammatory molecules (Hussain et al., 2016). Additionally, polyphenols can reduce oxidative damage by preventing LPS-induced nitric oxide synthase (iNOS) gene expression in cultured macrophages (Sarkar and Bhaduri, 2001). Umesalma and Sudhandiran (2010) found that both in vitro and in vivo studies have shown a connection between the release of prostaglandins and nitric oxide in the modulation of inflammation, which is significant because EA has been shown to significantly inhibit nitric oxide production by downregulating inducible nitric oxide synthase. In addition, the results revealed that TA as a positive control showed the highest inhibition of nitric oxide production. TA has been well known as an anti-inflammatory glucocorticoid medication inhibiting inflammatory cells and decreasing inflammatory mediator expression (Mark, 2021).
Chemical profiles are essential for authentication, quality assurance and obtaining convincing evidence, in addition to investigating phytochemical components and biological activity (Kumar, 2017; Cuadros-Rodríguez et al., 2021). Our result revealed that EA (at Rt = 33.16 min) was a major component found in the chromatographic fingerprint. Janpaijit et al. (2023) reported that EA inhibited the activation of nuclear factor kappa B (NF-κB) and iNOS function. In addition, the ethanolic seed extracts of C. nervosum demonstrated chemical compounds were brassitin, ferulic acid, EA, aurentiacin and alpinetin using liquid chromatography-mass spectrometry (LC-MS) (Janpaijit et al., 2023). Thus, EA might be used as a biological marker in quality control of product development.
The safety issue of promising bio-active compounds has been of great importance to be evaluated before human exposure. Therefore, HET-CAM was used to evaluate the safety profile of SE by observing hemorrhage, lysis, or coagulation that might occur after sample exposure. The results showed that SE produces no irritation on HET-CAM, implying its acceptable safety profile to be a bio-active ingredient in cosmetic products. In addition, SE should be evaluated for solubility and cytotoxicity and identify other unknown substances in the extract, including further evaluation for the safety and efficacy of the formulation in volunteers.
CONCLUSION
Our findings indicated that the C. nervosum extracts in forms of SE and WF contained high total phenolic and flavonoid contents corresponding to its antimicrobial effects against acne inducing bacteria and anti-oxidant activity. SE was the outstanding extract which also exerted an anti-inflammatory activity. Besides, a good safety profile of SE was revealed in HET-CAM. Thus, SE was a promising bio-active compounds for further developing into anti-acne cosmeceutical products.
ACKNOWLEDGMENTS
The authors are grateful to have been financially supported by the Teaching Assistant and Research Assistant scholarships from the Graduate School, Chiang Mai University and the Faculty of Pharmacy, Chiang Mai University, Thailand. In addition, the authors truly appreciate the Faculty of Dentistry, Chiang Mai University, Thailand and Dr. Phenphichar Wanachantararak for supporting the microbiology laboratory and all facilities. The English proof of this manuscript was executed by the International Relations Unit, Faculty of Pharmacy, Chiang Mai University.
AUTHOR CONTRIBUTIONS
Tharadon Khammitham carried out the experiments, assessed the statistical data and wrote the manuscript draft. Pimporn Leelapornpisid, Sunee Chansakaow, Warat Leelapornpisid and Worrapan Poomanee designed all of the experiments, supported all resources and revised the manuscript. The final manuscript has been read and approved by all authors.
CONFLICT OF INTEREST
The authors report they have no conflict of interest.
REFERENCES
Arsa, P. 2008. Chemical constituents and antibacterial activity on Propionibacterium acnes and Staphylococcus aureus of Cleistocalyx nervosum var. paniala seeds. Master’s Thesis, Chiang Mai University, Chiang Mai.
Bouarab-Chibane, L., Forquet, V., Lantéri, P., Clément, Y., Léonard-Akkari, L., Oulahal, N., Degraeve, P., and Bordes, C. 2019. Antibacterial properties of polyphenols: Characterization and QSAR (quantitative structure-activity relationship) models. Frontiers in Microbiology. 10: 829.
Chaiyana, W., Punyoyai, C., Somwongin, S., Leelapornpisid, P., Ingkaninan, K., Waranuch, N., Srivilai, J., Thitipramote, N., Wisuitiprot, W., Schuster, R. et al. 2017. Inhibition of 5α-reductase, IL-6 secretion, and oxidation process of Equisetum debile Roxb. ex Vaucher extract as functional food and nutraceuticals ingredients. Nutrients. 9(10): 1105.
Chariyakornkul, A., Juengwiroj, W., Ruangsuriya, J., and Wongpoomchai, R. 2022. Antioxidant extract from Cleistocalyx nervosum var. paniala pulp ameliorates acetaminophen-induced acute hepatotoxicity in rats. Molecules. 27(2): 553.
Cuadros-Rodríguez, L., Ortega-Gavilán, F., Martín-Torres, S., Arroyo-Cerezo, A., and Jiménez-Carvelo, A.M. 2021. Chromatographic fingerprinting and food identity/quality: Potentials and challenges. Journal of Agricultural and Food Chemistry. 69(48): 14428-14434.
Daglia, M. 2012. Polyphenols as antimicrobial agents. Current Opinion in Biotechnology. 23(2): 174-181.
Dhillon, K.S., and Varshney, K.R. 2013. Study of microbiological spectrum in acne vulgaris: An in vitro study. Scholars Journal of Applied Medical Sciences. 1(6): 724-727.
Dwyer, D. J., Kohanski, M. A., and Collins, J. J. 2009. Role of reactive oxygen species in antibiotic action and resistance. Current Opinion in Microbiology. 12(5): 482-489.
Freire, P.L., Stamford, T.C., Albuquerque, A.J., Sampaio, F.C., Cavalcante, H.M., Macedo, R.O., Galembeck, A., Flores, M.A. and Rosenblatt, A., 2015. Action of silver nanoparticles towards biological systems: Cytotoxicity evaluation using hen's egg test and inhibition of Streptococcus mutans biofilm formation. International Journal of Antimicrobial Agents. 45(2): 183-187.
Grange, P.A., Chéreau, C., Raingeaud, J., Nicco, C., Weill, B., Dupin, N., and Batteux, F. 2009. Production of superoxide anions by keratinocytes initiates P. acnes-induced inflammation of the skin. PLoS Pathogens. 5(7): e1000527.
Hussain, T., Tan, B., Yin, Y., Blachier, F., Tossou, M.C., and Rahu, N. 2016. Oxidative stress and inflammation: What polyphenols can do for us?. Oxidative Medicine and Cellular Longevity. 74(3): 2797.
ICH. 2022. International council for harmonization guideline Q2 (R2) on validation of analytical procedures: Text and methodology. European Medicines Agency.
Janpaijit, S., Sillapachaiyaporn, C., Theerasri, A., Charoenkiatkul, S., Sukprasansap, M., and Tencomnao, T. 2023. Cleistocalyx nervosum var. paniala berry seed protects against TNF-α-stimulated neuroinflammation by inducing HO-1 and suppressing NF-κB mechanism in BV-2 microglial cells. Molecules. 28(7): 3057.
Kaczorová, D., Karalija, E., Dahija, S., Bešta-Gajević, R., Parić, A., and Ćavar Zeljković, S. 2021. Influence of extraction solvent on the phenolic profile and bioactivity of two Achillea species. Molecules. 26(6): 1601.
Kardeh, S., Moein, S.A., Namazi, M.R., and Kardeh, B. 2019. Evidence for the important role of oxidative stress in the pathogenesis of acne. Galen Medical Journal. 8: e1291.
Keepers, T.R., Gomez, M., Celeri, C., Nichols, W.W., and Krause, K.M. 2014. Bactericidal activity, absence of serum effect, and time-kill kinetics of ceftazidime-avibactam against β-lactamase-producing Enterobacteriaceae and Pseudomonas aeruginosa. Antimicrobial Agents and Chemotherapy. 58(9): 5297-5305.
Koreck, A., Pivarcsi, A., Dobozy, A., and Kemény, L. 2003. The role of innate immunity in the pathogenesis of acne. Dermatology. 206(2): 96-105.
Kumar, B.R. 2017. Application of HPLC and ESI-MS techniques in the analysis of phenolic acids and flavonoids from green leafy vegetables (GLVs). Journal of Pharmaceutical Analysis. 7(6): 349-364.
Lee, J., Durst, R.W., and Wrolstad, R.E. 2005. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: collaborative study. Journal of AOAC International. 88(5): 1269-1278.
Lertsatitthanakorn, P., Taweechaisupapong, S., Arunyanart, C., Aromdee, C., and Khunkitti, W. 2010. Effect of citronella oil on time kill profile, leakage and morphological changes of Propionibacterium acnes. Journal of Essential Oil Research. 22(3): 270-274.
Likittrakulwong, W., Chanburee, S., Kitpot, T., Ninjiaranai, P., and Pongpamorn, P. 2023. Phytochemical properties, in vitro antimicrobial, and bioactive compounds of banana peel extractions using GC-MS. Natural and Life Sciences Communications. 22(2): e2023021.
Maisuthisakul, P., Suttajit, M., and Pongsawatmanit, R. 2007. Assessment of phenolic content and free radical-scavenging capacity of some Thai indigenous plants. Food Chemistry. 100(4): 1409-1418.
Maneechai, P., Leelapornpisid, P., and Poomanee, W. 2023. Multifunctional biological activities and cytotoxic evaluation of Bouea macrophylla for cosmetic applications. Natural and Life Sciences Communications. 22(2): e2023030.
Marković, Z., Milenković, D., Đorović, J., Dimitrić Marković, J. M., Lučić, B., and Amić, D. 2013. A DFT and PM6 study of free radical scavenging activity of ellagic acid. Monatshefte für Chemie-Chemical Monthly. 144: 803-812.
Mark, G. 2021. Triamcinolone Acetonide, Triamcinolone Hexacetonide, Triamcinolone Diacetate. Papich Handbook of Veterinary Drugs (Fifth Edition).
Mawardi, P., Ardiani, I., Primisawitri, P.P., and Nareswari, A. 2021. Dual role of Cutibacterium acnes in acne vulgaris pathophysiology. Bali Medical Journal. 10(2): 486-490.
Mustafa, R.A., Hamid, A.A., Mohamed, S., and Bakar, F.A. 2010. Total phenolic compounds, flavonoids, and radical scavenging activity of 21 selected tropical plants. Journal of Food Science. 75(1): 28-35.
Poomanee, W., Chaiyana, W., Mueller, M., Viernstein, H., Khunkitti, W., and Leelapornpisid, P. 2018. In-vitro investigation of anti-acne properties of Mangifera indica L. kernel extract and its mechanism of action against Propionibacterium acnes. Anaerobe. 52: 64-74.
Poomanee, W., Chaiyana, W., Intasai, N., and Leelapornpisid, P. 2015. Biological activities and characterization of the pod extracts from Sompoi (Acacia concinna linn) grown in northern Thailand. International Journal of Pharmacy and Pharmaceutical Sciences. 7: 237-241.
Poontawee, W., Natakankitkul, S., and Wongmekiat, O. 2016. Protective effect of Cleistocalyx nervosum var. paniala fruit extract against oxidative renal damage caused by cadmium. Molecules. 21(2): 133.
Prasanth, M.I., Sivamaruthi, B.S., Sukprasansap, M., Chuchawankul, S., Tencomnao, T., and Chaiyasut, C. 2020. Functional properties and bioactivities of Cleistocalyx nervosum var. paniala berry plant: A review. Food Science and Technology. 40: 369-373.
Qin, M., Landriscina, A., Rosen, J.M., Wei, G., Kao, S., Olcott, W., Agak, G.W., Paz, K.B., Bonventre, J., Clendaniel, A. et al. 2015. Nitric oxide-releasing nanoparticles prevent Propionibacterium acnes-induced inflammation by both clearing the organism and inhibiting microbial stimulation of the innate immune response. Journal of Investigative Dermatology. 135(11): 2723-2731.
Rowe, R.C., Sheskey, P.J., and Quinn, M.E. 2009. Handbook of pharmaceutical excipients 6th edition pharmaceutical press. London, England.
Sahu, G.K., Sharma, H., Gupta, A., and Kaur, C.D. 2015. Advancements in microemulsion based drug delivery systems for better therapeutic effects. International Journal of Pharmaceutical Sciences and Developmental Research. 1(1): 8-15.
Sarkar, A., and Bhaduri, A. 2001. Black tea is a powerful chemopreventor of reactive oxygen and nitrogen species: Comparison with its individual catechin constituents and green tea. Biochemical and Biophysical Research Communications. 284(1): 173-178.
Sharma, J.N., Al-Omran, A., and Parvathy, S.S. 2007. Role of nitric oxide in inflammatory diseases. Inflammopharmacology. 15(6): 252-259.
Sivasankar, C., Maruthupandiyan, S., Balamurugan, K., James, P. B., Krishnan, V., and Pandian, S. K. 2016. A combination of ellagic acid and tetracycline inhibits biofilm formation and the associated virulence of Propionibacterium acnes in vitro and in vivo. Biofouling. 32(4): 397-410.
Srihaphon, K., Wongwat, T., Lamlertthon, S., and Pitaksuteepong, T. 2020. Investigation on the potential application of Morus alba stem extract for inflammatory acne vulgaris. Songklanakarin Journal of Science and Technology. 42(6): 1319-1325.
Sukprasansap, M., Chanvorachote, P., and Tencomnao, T. 2017. Cleistocalyx nervosum var. paniala berry fruit protects neurotoxicity against endoplasmic reticulum stress-induced apoptosis. Food and Chemical Toxicology. 103: 279-288.
Thielitz, A., Helmdach, M., Röpke, E.M., and Gollnick, H. 2001. Lipid analysis of follicular casts from cyanoacrylate strips as a new method for studying therapeutic effects of antiacne agents. British Journal of Dermatology. 145(1): 19-27.
Torres-Rodríguez, M.L., García-Chávez, E., Berhow, M., and De Mejia, E.G. 2016. Anti-inflammatory and anti-oxidant effect of Calea urticifolia lyophilized aqueous extract on lipopolysaccharide-stimulated RAW 264.7 macrophages. Journal of Ethnopharmacology. 188: 266-274.
Tsao, R. 2010. Chemistry and biochemistry of dietary polyphenols. Nutrients. 2(12): 1231-1246.
Umesalma, S., and Sudhandiran, G. 2010. Differential inhibitory effects of the polyphenol ellagic acid on inflammatory mediators NF-kappaB, iNOS, COX-2, TNF-alpha, and IL-6 in 1, 2-dimethylhydrazine-induced rat colon carcinogenesis. Basic & Clinical Pharmacology & Toxicology. 107(2): 650-655.
Wayne, P.A. 2020. Clinical and laboratory standards institute: Performance standards for antimicrobial susceptibility testing: 30th informational supplement. CLSI supplement M100.
Wood, T.K., Knabel, S.J., and Kwan, B.W. 2013. Bacterial persister cell formation and dormancy. Applied and Environmental Microbiology. 79(23): 7116-7121.
Yang, G., Lee, K., Lee, M., Ham, I., and Choi, H.Y. 2012. Inhibition of lipopolysaccharide-induced nitric oxide and prostaglandin E2 production by chloroform fraction of Cudrania tricuspidata in RAW 264.7 macrophages. BMC Complementary and Alternative Medicine. 12(1): 1-7.
Zhang, Q.W., Lin, L.G., and Ye, W.C. 2018. Techniques for extraction and isolation of natural products: A comprehensive review. Chinese Medicine. 13: 1-26.
OPEN access freely available online
Natural and Life Sciences Communications
Chiang Mai University, Thailand. https://cmuj.cmu.ac.th
Tharadon Khammitham1, Pimporn Leelapornpisid1, 2, Sunee Chansakaow1, Warat Leelapornpisid3, and Worrapan Poomanee1, 2, *
1 Department of Pharmaceutical Sciences, Faculty of Pharmacy, Chiang Mai University, Chiang Mai 50200, Thailand.
2 Innovation Center for Holistic Health, Nutraceuticals, and Cosmeceuticals, Faculty of Pharmacy, Chiang Mai University, Chiang Mai 50200, Thailand.
3 Department of Restorative Dentistry, Faculty of Dentistry, Chiang Mai University, Chiang Mai 50200, Thailand.
Corresponding author: Worrapan Poomanee E-mail: worrapan.p@cmu.ac.th
Total Article Views
Editor: Nisit Kittipongpatana
Chiang Mai University, Thailand
Article history:
Received: June 14, 2023;
Revised: August 23, 2023;
Accepted: August 24, 2023;
Online First: August 30, 2023

