
Fabrication of Novel Floating 3D-Printed Devices with Adjustable Releasing Channels for Controlled Release
Thapakorn Charoenying, Prasert Akkaramongkolporn, Praneet Opanasopit, Prasopchai Patrojanasophon and Supusson Pengnam*Published Date : August 25, 2023
DOI : https://doi.org/10.12982/NLSC.2023.063
Journal Issues : Number 4, October-December 2023
Abstract Gastro-retentive drug delivery systems (GRDDS) have been used to improve the therapeutic efficacy of drugs with narrow absorption windows, unstable in alkaline pH, soluble in acidic conditions, and active locally in the stomach. This study aimed to produce a novel floating three-dimensional (3D)-printed device (F3D) from polylactic acid filaments using a fused deposition modeling 3D printer. There were two parts of F3D: a cap with an air chamber for floating property and a body with 6 channels for controlled release. The device was designed to have adjustable channels for the releasing control and gastric retention of Bromhexine, carvedilol, and theophylline. The releasing channels could be adjusted by rotating the base to open or close the number of releasing channels as 3 levels (2, 4, and 6 channels). Morphology, weight variation, ex vivo floating time, and drug release characteristics were examined. The F3D had a smooth texture with a narrow SD value of weight variation and actual size, suggesting that the F3D had high accuracy and consistent fabrication using 3D printing technology. All tablets incorporated F3D was able to float for at least 24 h. Decreasing the number of channels on the devices led to the sustained release of drugs following 2> 4> 6 channels. Hence, F3D could be useful in controlling drug release for various commercial tablets.
Keywords: 3D-printed device, Floating device, Fused deposition modeling, Gastroretentive drug delivery systems, controlled release dosage form
Funding: This research is funded by the National Research Council of Thailand (NRCT): N42A650551, N42A660847, and the Commission of Higher Education (Thailand).
Citation: Charoenying, T., Akkaramongkolporn, P., Opanasopit, P., Patrojanasophon, P., and Pengnam, S. 2023. Fabrication of novel floating 3D-printed devices with adjustable releasing channels for controlled release. Natural and Life Sciences Communications. 22(4): e2023063.
INTRODUCTION
Oral formulations are the most commonly used dose form for human administration. Nevertheless, the use of conventional oral dosage forms could be limited by concerns such as drug solubility in base/acid, which results in only a low amount of the drug being available at the site of action or absorption. Low bioavailability is one of the main hindrances since humans have a short gastric-emptying time (Mandal et al., 2016; Nayak et al., 2010). Recently, Various dosage forms for gastroretentive drug delivery systems (GRDDS) have been developed, such as floating systems, polymer swelling systems, high-density systems, mucoadhesion systems, magnetic force systems, floating microsphere systems, and effervescence systems (Mandal et al., 2016). The floating systems are low-density systems with a density less than that of the gastric fluid in order to float on the gastric fluid and remain in the stomach to prolong the release of drugs, resulting in increased bioavailability (Chai et al., 2017; Pawar et al., 2011). However, the crucial limitation of floating systems was the material selection providing lower density than the gastric fluid.
Three-dimension (3D) printing technology has attracted massive attention from pharmaceutical fields due to producing complex internal structures to overcome the limitation of material selection for manufacturing floating systems (Charoenying et al., 2020a). Currently, there are many types of 3D printers, such as binder jetting, digital light processing (DLP), stereolithography (SLA), direct metal laser sintering (DMLS), selective laser sintering (SLS), selective laser melting (SLM), material jetting (MJ), fused deposition modeling (FDM), etc. (Redwood et al., 2017). FDM 3D printers are the most popular because of their established quality criteria, printing precision, and cost-effectiveness (Vaz et al., 2021). Previously, FDM 3D printer was employed to fabricate the 3D printed device for GRDDS. Fu and coworkers developed a tablet-in-device (TiD) system containing a riboflavin sustained release (SR) tablet made of polylactic acid (PLA). This device was designed with two chambers: one containing a tablet and the other an air chamber for buoyancy. In comparison, this TiD could float in a rabbit's stomach for more than three days (Fu et al., 2018). Moreover, Huanbutta and coworkers also fabricated floating tablet housing with PLA to contain metronidazole in-house tablet. The result shows zero‑order drug release kinetics (Huanbutta et al., 2021). PLA can be degraded in a natural environment and eliminated from the body (Qi et al., 2017; Song et al., 2009). Charoenying et al. fabricated domperidone tablet-incorporated 3D-printed devices with PLA and polyvinyl alcohol (PVA) to control the release and floating time, respectively. This study suggested that the dissolving property of PLA was slower than PVA (Charoenying et al., 2020b). Moreover, Huanbutta and coworkers investigated PVA devices to contain metronidazole in-house tablets. The result exhibited that PVA devices observed an enlarger pore in the dissolution method (Huanbutta et al., 2019). Thus, the PLA was considered to fabricate the floating 3D printed device (F3D) to control release in this study because it maintains the configuration throughout the experiment.
F3D was designed to have an internal air chamber for floating properties. The previous 3D-printed device was fabricated to fit only one type of drug tablet. Thus, the F3D was designed to incorporate various commercial tablets and adjustable releasing channels to modify the drug release rate. Bromhexine and carvedilol were selected to represent the immediate release of commercial tablets. The theophylline tablet represents the sustained-release formulation. The model drugs were selected due to poorly soluble in alkaline pH (Harikumar et al., 2016; Srikanth Meka et al., 2016; Tripathi et al., 2019). The morphology, weight variation, ex vivo floating time, and drug release characteristics were examined.
MATERIALS AND METHODS
Materials
Carvedilol 6.25 mg tablets (Caraten®) were a Berlin Pharmaceutical Industry Co.,Ltd., (Bangkok, Thailand) product. Bromhexine 8 mg tablets (Bisolvon®) were supplied from Sanofi-Aventis (Thailand) Ltd., and Theophylline SR 200 mg tablets (Nuelin®) were purchased from INOVA pharma (Thailand) Co., Ltd. Polylactic acid (PLA) filaments (1.75-diameter) were supplied from Shenzhen Yun Industrial Co., Ltd. (Shenzhen, China). Hydrochloric acid fuming (HCl) and orthophosphoric acid were provided by Merck KGaA (Darmstadt, Germany). Sodium chloride (NaCl) and triethylamine were procured from Sigma-Aldrich (Steinheim, Germany). Acetonitrile was provided by Honeywell International Inc. (New Jersey, USA). Other chemicals and solvents were supplied from commercially available sources.
Design of F3D
F3D was designed using an Autodesk® Fusion 360TM Design program (V. 2.0.15509). F3D has two parts consisting of (1) a cap with an internal air chamber for floating property (Figure 1B) and 6 channels for controlled release (Figure 1A), and (2) a body with a base and 2-sides wall (Figure 1C). The body of F3D can be rotated to adjust open channel levels in 3 levels; 2, 4, and 6 open channels (Figure 1F-H). All the dimensions of F3D are illustrated in Figure1.
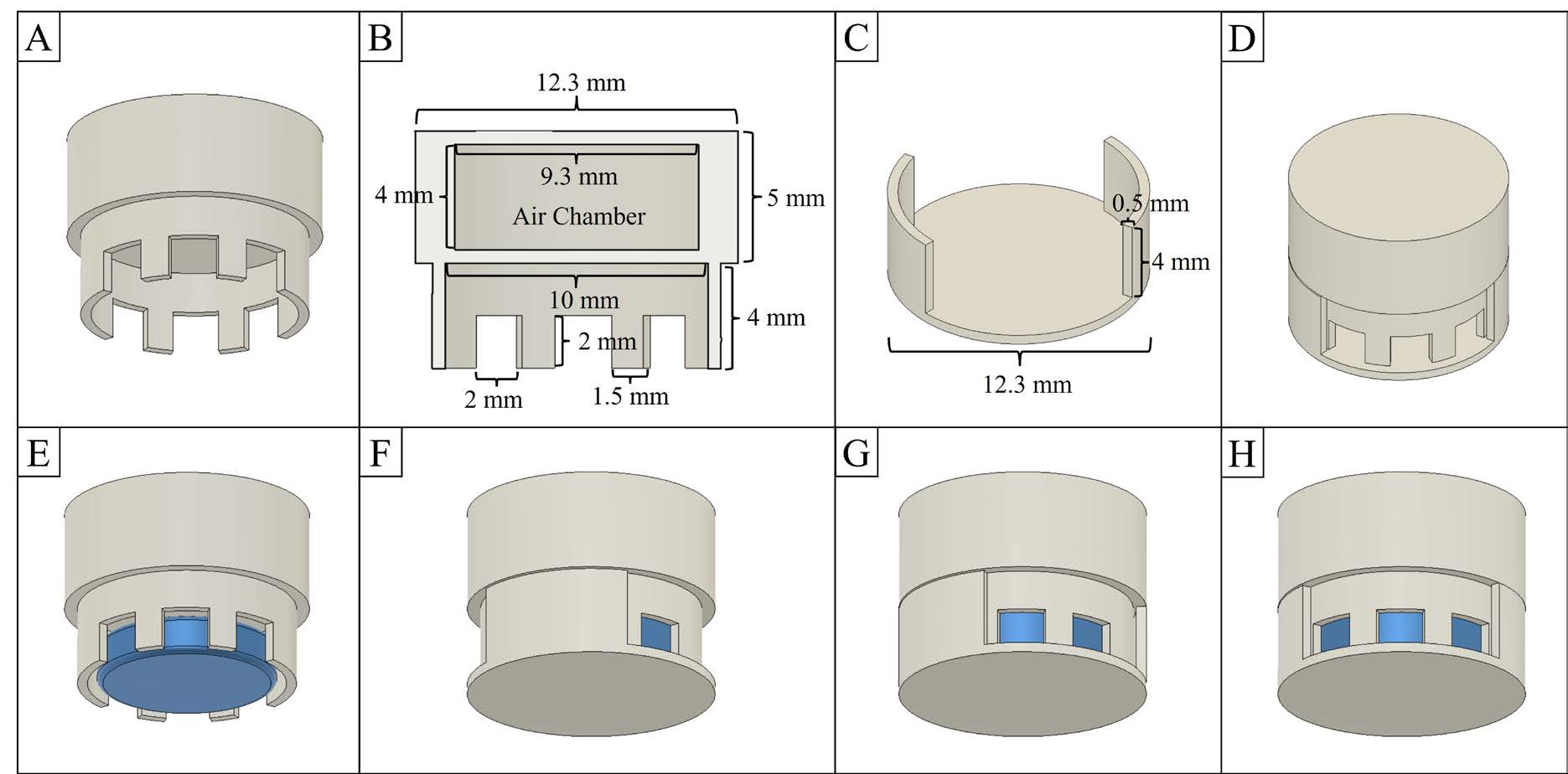
Figure 1. Illustrative designs of F3D; (A) the cap. (B) The cap's dimension and vertical cut view show the internal air chamber. (C) The body's dimension (D) Assemble of F3D. (E) The location of a drug tablet (blue). The open channel levels of F3D: (F) 2, (G) 4, and (H) 6 channels.
Printer setting for F3D fabrication
This study employed an FDM 3D printer (Prusa i3 MK3, Prusa Research S.R.O., Prague, Czech Republic) to fabricate F3D using PLA filament. A slicer software (PrusaSlicer V.2.3.0) was employed for printer settings, as listed in Table 1. The extruder and bed temperature were set following the printing filament recommendations. In-fill density was set as 100% to avoid other internal air chambers that affect the device's density. Other parameters were set as default.
Table 1. The settings of the FDM printer.
|
Parameters |
Setting conditions |
|
Extruder temperature |
215 °C |
|
Bed temperature |
60 °C |
|
Nozzle diameter |
0.4 mm |
|
Layer height |
0.15 mm |
|
Vertical shells |
2 |
|
Infill density |
100% |
|
Infill pattern |
Rectilinear |
|
Speed while printing |
45 mm/s |
|
Speed while traveling |
180 mm/s |
|
Unloaded |
70 |
|
Loaded |
100 |
Characterization of the F3D
Morphology of the F3D and commercial drug tablet
The morphology of the F3D was investigated by an Apple iPhone 12 Pro Max camera. In addition, a digital clipper was employed to determine the actual dimension of the F3D (Zhejiang Deqing Syntek Electronic Technology Co., Ltd, Deqing, China).
Weight variation of the F3D
The accurate weight of the F3D was determined by weighing 20 pieces of each part separately with Sartorius® series- CP224S analytical balance (Data Weighing Systems, Inc., Illinois, USA). Weight variation was recorded as a mean ± standard deviation (SD).
Ex vivo floating time of F3D
Drug tablets were placed into the body part and assembled with the cap part. The floating ability of assembled F3D was evaluated using a USP dissolution paddle apparatus. Each vessel contained 900 ml of simulated gastric fluid (SGF) (0.1 N HCl, pH 1.2) as the dissolving media. A digital camera (Sony Camera HANDYCAM® PJ420) was used to measure the floating lag time (FLT) and total floating time (TFT). The refloating ability of the floatable F3D was tested in a beaker containing 900 mL of SGF at 37 ± 0.5 °C with stirring at 75 rpm. The F3D was submerged in SGF for 5 seconds per hour with a glass rod for 24 h.
The drug release characteristics
The dissolution of drug tablets and drug tablet-incorporated F3Ds with different open channel levels, 2, 4, and 6 channels, were investigated using a USP dissolution paddle apparatus (ERWEKA GmbH, Germany). Each vessel contained 900 ml of SGF (0.1 N HCl, pH 1.2) as the dissolving media at 37 ± 0.5 °C with 75 rpm of paddle rotation speed to mimic the stomach environment. The dissolution medium was withdrawn at predetermined time intervals (5 min, 0.25, 0.5, 1, 1.5, 2, 4, 6, 8, 12, and 24 h) and immediately replaced with a fresh medium to maintain the sink condition. A 0.45-micron nylon filter was used to filter the withdrawn samples.
Drug content analysis
Bromhexine
High-performance liquid chromatography (HPLC) (Model: 1,260 infinitely series, Agilent Technologies Inc., California, USA) connected to a reversed-phase C-18 column (Phenomenex®, 5 μm particle size, 4.6 mm × 15 cm) was employed to quantify the released amount of Bromhexine. The injection volume was set at 10 μL. The pH of the mobile phase consisting of acetonitrile and 0.5% orthophosphoric acid, was adjusted with triethylamine (80:20 v/v) to obtain pH 7.0. The flow rate was set at 1.0 ml/min. The eluent was detected with a UV detector operated at a wavelength of 248 nm.
Theophylline
The amount of theophylline was analyzed using a UV-vis absorption spectroscopy (Victor Nivo: PerkinElmer, Finland) at an absorbance of 272 nm. The cumulative release graph was also plotted.
Carvedilol
A UV-vis spectrophotometer was employed to analyze the carvedilol content from the withdrawn dissolution medium at 285 (A285) and 380 (A380) nm absorbance. The corrected absorbance (ACORR) was calculated following equation (1).
ACORR = A285 - A380 (1)
Drug release kinetics of F3D
The dissolution profiles of the drug tablet-incorporated F3D were fit to the zero-order, first-order, and Higuchi kinetics models. The fitting data were reported with a correlation coefficient (R2). The higher R2 values represented better fits between the data and kinetic equations.
Statistics analysis
All experiments were conducted three times. The findings were reported as the mean ± SD. In addition, the Student's t-test was employed to examine statistically significant differences at P < 0.05.
RESULTS
Characterization of the F3D
Morphology of the F3D and commercial drug tablet
The morphology of the F3D is illustrated in Fig 2. The appearance was similar to the 3D model design. F3D presented white from the initial color of the PLA filament. The texture of F3D was smooth with a stripe pattern. Figure 3 shows the F3D dimensions, and the actual size of the F3D is depicted in Table 2. The actual size of commercial drug tablets is listed in Table 3.
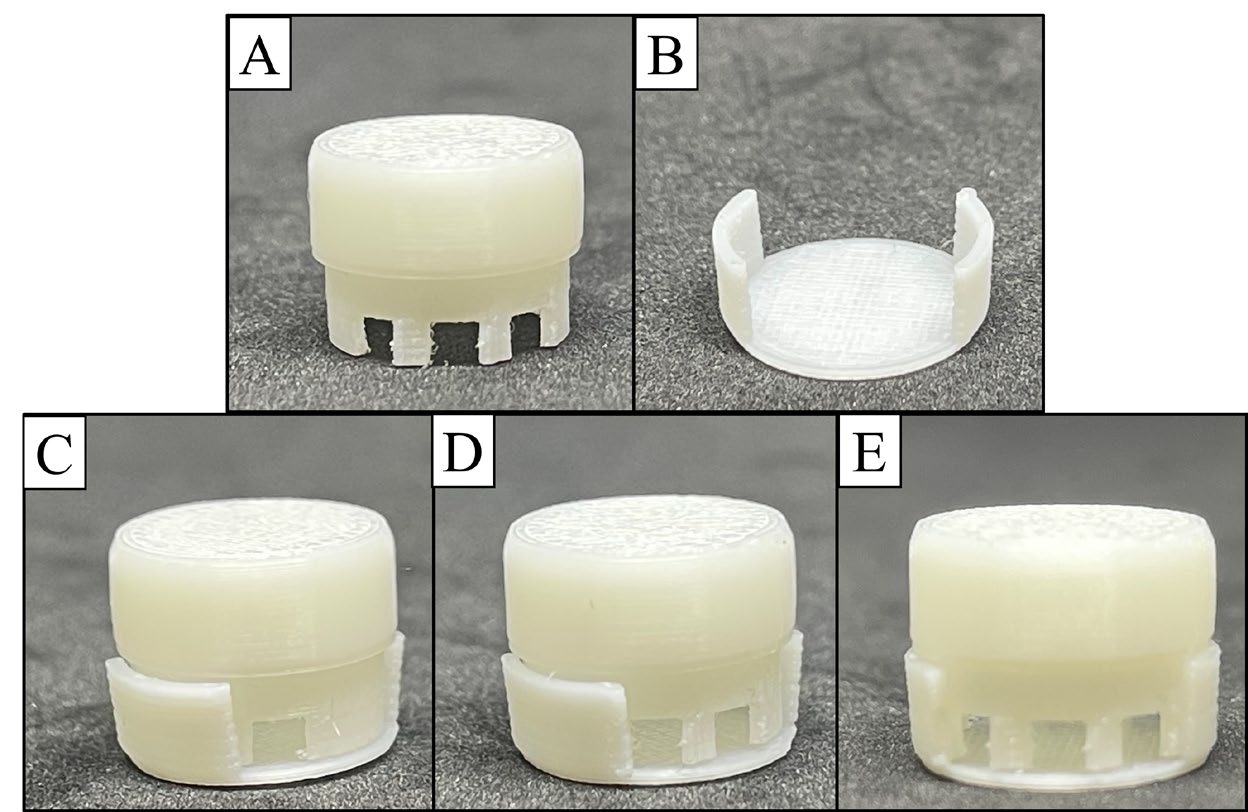
Figure 2. The appearance of (A) the cap and (B) the body of F3D. Assembled F3D with 3 open channel levels: (F) 2, (G) 4, and (H) 6 channels.

Figure 3. The dimensions of (A) the cap and (B) the body of F3D.
Table 2. The actual size of F3D.
|
Part of F3D |
Dimensions in Figure 3 |
Actual size (mm) |
|
Cap |
A |
12.31 ± 0.02 |
|
|
B |
5.00 ± 0.01 |
|
|
C |
4.00 ± 0.02 |
|
|
D |
10.00 ± 0.01 |
|
|
E |
2.01 ± 0.02 |
|
|
F |
2.00 ± 0.02 |
|
|
G |
1.51 ± 0.02 |
|
Body |
A |
12.30 ± 0.01 |
|
|
B |
4.01 ± 0.02 |
Table 3. The actual size of commercial drug tablets.
|
Commercial drug tablet |
Diameter (mm) |
Thickness (mm) |
|
Carvedilol |
7.07 ± 0.01 |
1.92 ± 0.02 |
|
Bromhexine |
7.08 ± 0.02 |
2.41 ± 0.01 |
|
Theophylline |
9.02 ± 0.02 |
3.65 ± 0.02 |
Weight variation of the F3D
The 20 pieces of the cap and the body of F3D were individually weighed using an analytical balance. The weight variation result of the F3D is listed in Table 4.
Table 4. The weight variation of F3D.
|
Part of F3D |
Average weight (mg) |
|
Cap |
499.78 ± 2.40 |
|
Body |
119.81 ± 1.44 |
Ex vivo floating time of F3D
All F3Ds floated immediately after placing into the vessel (FLT = 0 min). The TFT results showed that the F3D could float over 24 h. The floating characteristics of F3D are illustrated in Figure 4. All drug tablets were submerged in the medium throughout the experiment.

Figure 4. Illustrative floating characteristics of F3D containing (A) carvedilol, (B) bromhexine, and (C) theophylline tablet with 6 open channels.
The drug release characteristics of tablet-incorporated F3D
Carvedilol
The cumulative release of carvedilol is illustrated in Figure 5A. The result showed that the carvedilol tablet was immediately released around 97% at 0.5 h. On the other hand, the carvedilol tablet-incorporated F3D exhibited a slower release rate when compared to the conventional tablet. Carvedilol tablet-incorporated F3D with 4 and 6 open channels achieved ~90% cumulative release at 4 and 2 h, respectively, while the F3D with 2 open channels had the slowest release rate, reaching ~90% cumulative release at 8 hours.
Bromhexine
Figure 5B shows a release profile of a bromhexine tablet and the Bromhexine tablet-incorporated F3D. The bromhexine table was immediately released, which was 95% at 0.5 h. In contrast, Bromhexine tablet-incorporated F3D exhibited a sustained release profile. The bromhexine tablet incorporated F3D with 4 and 6 open channels achieved around 97% cumulative release at 12 and 6 h, respectively. In addition, The F3D with 2 open channels also showed the slowest drug release rate, reaching ~100% cumulative release at 24 h.
Theophylline
Theophylline tablets are a sustained-release formulation. In Figure 5C, the release profile of the theophylline tablet exhibited a sustained release profile which released 92% at 12 h. In addition, the theophylline tablet-incorporated F3D with 2, 4, and 6 open channels achieved ~35%, ~52%, and ~67% cumulative release at 24 h, respectively. Therefore, the F3D could reduce the release rate of theophylline tablets.
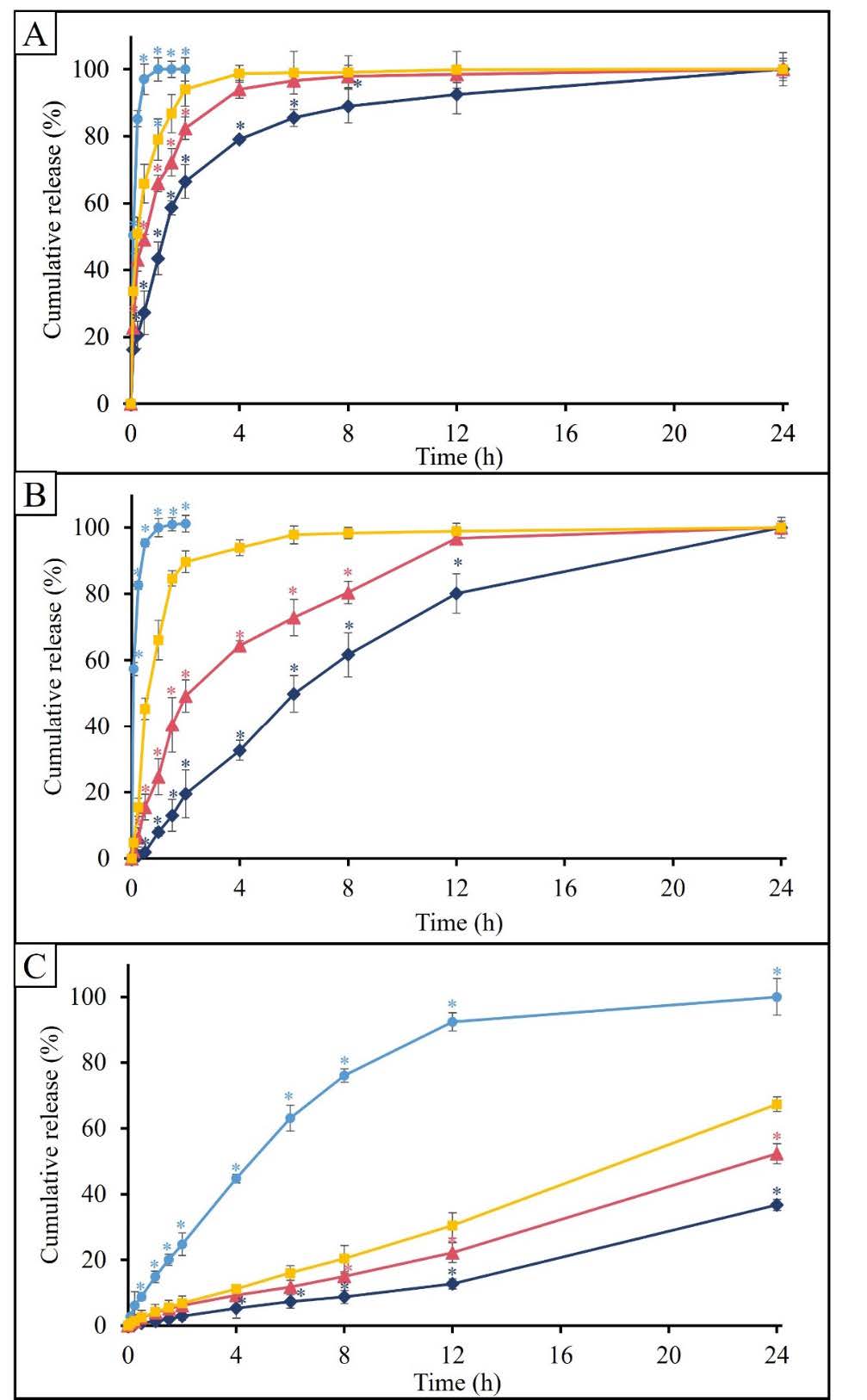
Figure 5. The cumulative release graphs of (A) carvedilol, (B) bromhexine, and (C) theophylline. Original dosage form of carvedilol, Bromhexine, and theophylline (control) (  ). Drug incorporated F3D with 2 (
). Drug incorporated F3D with 2 ( ), 4 (
), 4 ( ), and 6 (
), and 6 ( ) open channels. * Statistically significant difference at P <0.05 from the drug tablet-incoperaterd F3D with 6 open channels.
) open channels. * Statistically significant difference at P <0.05 from the drug tablet-incoperaterd F3D with 6 open channels.
Drug release Kinetics of F3D
The results of kinetic release are listed in Table 5. The in vitro release profiles of the carvedilol tablet incorporated F3D with 2, 4, and 6 open channels were close to 100% at 8, 4, and 2 h, respectively. In addition, the in vitro release profiles of the bromhexine tablet incorporated F3D with 4 and 6 open channels were close to 100% at 12 and 6 h, respectively. Therefore, the release kinetics should be calculated within the time period of drug release. The findings showed that the release kinetic of carvedilol and Bromhexine tablet-incorporated F3D was fit with first-order. On the other hand, the theophylline tablet-incorporated F3D was fit with zero-order.
Table 5. The release kinetics of the drug tablet-incorporated F3D with various open channels.
|
Drug |
Number of open channels |
Zero-order (R2) |
First-order (R2) |
Higuchi model (R2) |
|
Carvedilol |
- 2 |
0.7873 0.7667 |
0.9828* 0.9471* |
0.7731 0.9307 |
|
|
4 |
0.7205 |
0.9889* |
0.8965 |
|
|
6 |
0.7626 |
0.9903* |
0.9708 |
|
Bromhexine |
- 2 |
0.7417 0.8809 |
0.9770* 0.9874* |
0.8365 0.9700 |
|
|
4 |
0.8576 |
0.9871* |
0.9691 |
|
|
6 |
0.6190 |
0.9113* |
0.8454 |
|
Theophylline |
- |
0.9912* |
0.9750 |
0.9765 |
|
|
2 |
0.9743* |
0.9527 |
0.8263 |
|
|
4 |
0.9883* |
0.9610 |
0.9863 |
|
|
6 |
0.9958* |
0.9574 |
0.8856 |
Note: * The highest R2 value
DISCUSSION
The F3D was designed to prolong the drug release in the stomach of the narrow absorption window drugs. The F3D with adjustable open channels provided the floating properties and the ability to sustain the drug release. In addition, the release channels could be adjusted for 3 levels (2, 4, and 6 open channels) depending on the different characteristics of commercial drug tablets. Thus, the adjustable releasing channels can be modified to suit the drug release rate of other commercial tablets.
The obtained F3D had a configuration similar to the designed 3D model. The stripe pattern of F3D was obtained from the principle of an FDM 3D printer that prints the filament layer-by-layer (Goyanes et al., 2014; Fafenrot et al., 2017; Charoenying et al., 2020a). The narrow SD values of actual dimension and weight variation results indicated that the FDM 3D printer was a consistent and accurate technology. These results corresponded with previous articles that reported a narrow SD value of actual dimension and weight variations (Charoenying et al., 2020b; Chareonying et al., 2022). Moreover, the dimension accuracy could affect the drug release consistency because the channel size could alter the drug release rate.
The drug tablet-incorporated F3D immediately floated after placing into the vessel because the F3D consisting of an internal air chamber in the cap part resulted in a lower density of drug tablet-incorporated compared to SGF. Previous literature reported the FLT was less than 0 min due to a low-density property of the whole formulation (Fu et al., 2018). The TFT was more than 24 h due to the slow degradation of PLA. Moreover, drug tablets in F3D were always submerged in the medium throughout the experiment because F3D design had an air chamber at the top. Therefore, The air chamber could keep the device in an upright position all the time.
The dissolution of drug-incorporated F3D exhibited a sustained release profile, although the original commercial tablet was an immediate-release dosage form. Carvedilol and bromhexine commercial tablets showed similar immediate-release profiles. If more frequent sampling was given, the immediate-release profiles of carvedilol and bromhexine commercial tablets should differ due to the difference in excipient and active ingredients. Thus, carvedilol and bromhexine incorporated in F3D exhibited different release profiles. This finding was in agreement with Noyes-Whitney's equation which states that the dissolution rate is proportional to the surface area exposed to the medium (Hattori et al., 2012). Furthermore, according to a recent article, the hole width of PLA devices was varied. The results showed that the big hole width exhibited greater drug release than the small hole width (Charoenying et al., 2023; Charoenying et al., 2020b). This could be concluded that the number of open channels was the most important factor influencing drug release. Nonetheless, the release profile of theophylline tablet, sustained-release formulation incorporated in F3D, was also in agreement with Noyes-Whitney's equation. These findings can be concluded that the decreasing number of channels on the devices led to the sustained release of drugs following 2> 4> 6 open channels. Previously, the devices were printed with unchanged hole sizes unless redesigned and printed (Huanbutta et al., 2019; Shin et al., 2019; Huanbutta et al., 2021; Charoenying et al., 2023). It can be concluded that this is the first study to fabricate PLA floating devices with adjustable releasing channels for controlled release. After 24 h, the F3D remained original shape due to its hydrophobic property and ability to maintain controlled release property. PLA is used as a biodegradable and biocompatible material. Shin and coworkers fabricated a PLA device as GRDDS, which can be eliminated from Beagle dogs at 48 h (Shin et al., 2019).
The release rate could be decreased by reducing the contract surface area of tablets to medium using the F3D adjustable releasing channels. The release kinetics of commercially sustained release dosage form (theophylline) fit well with zero-order kinetic because the release rate is controlled by release excipients. However, the release characteristic of theophylline tablet-incorporated F3D was not changed because of controlled release excipients in the drug tablet. The carvedilol and bromhexine fit well with first-order kinetic because the drug's release rate depends on the initial drug concentration. These results disagree with the previous article. The 3D-printed device was designed to fit completely with a carvedilol tablet size. The release kinetics of this system fits well with zero-order kinetic since only one side of the tablet contracted with the medium throughout the dissolution experiment (Chareonying et al., 2022). In comparison, the F3D was designed to incorporate any drug tablets. Therefore, free space between the side of the drug tablet and F3D allowed the medium to flow through the free space. The diameter of carvedilol and bromhexine tablets was similar, whereas the tablets' thickness was slightly different. However, the tablet size was not the only factor affecting the release profile. The dissolution rate of the drug and other excipients of the commercial tablets should be considered. Finally, F3D exhibited sustained drug release because different angles of open channels hindered the directional flow of the medium.
CONCLUSION
In this study, the F3D was successfully fabricated using FDM 3D printer with PLA filament. The F3D had a smooth texture with a narrow SD value of weight variation and actual size, suggesting that the F3D had high accuracy and consistent fabrication using 3D printing technology. The release kinetic profile of the immediate-release formulation-incorporated F3D (carvedilol and bromhexine tablets) fit well with first-order kinetic. On the other hand, a sustained-release formulation-incorporated F3D (theophylline tablet) fits well with zero-order kinetic. All tablets incorporated F3D was able to float for at least 24 h. Decreasing the number of channels on the devices led to the sustained release of drugs following 2> 4> 6 open channels. Hence, F3D might be useful for drug release control of various commercial tablets.
ACKNOWLEDGMENTS
The authors thank the Pharmaceutical Development of Green Innovations Group (PDGIG) and the Faculty of Pharmacy, Silpakorn University, Nakhon Pathom, for providing instruments.
AUTHOR CONTRIBUTIONS
Thapakorn Charoenying conducted all of the experiments and wrote the manuscript. Prasert Akkaramongkolporn assisted in conducting the experiments and performed the statistical analysis. Praneet Opanasopit participated in the research design and reviewed and edited the manuscript. Prasopchai Patrojanasophon performed data analysis and reviewed and edited the manuscript. Supusson Pengnam designed the experiments, contributed data visualization, reviewed and edited the manuscript.
CONFLICT OF INTEREST
The authors declare that they hold no competing interests.
REFERENCES
Chai, X., Chai, H., Wang, X., Yang, J., Li, J., Zhao, Y., Cai, W., Tao, T., and Xiang, X., 2017. Fused deposition modeling (FDM) 3D printed tablets for Intragastric floating delivery of domperidone. Scientific Reports. 7: 2829.
Chareonying, T., Akkaramongkolporn, P., and Opanasopit, P., 2022. Development of floating 3D-printed devices for carvedilol tablet. Key Engineering Materials. 914: 45-51.
Charoenying, T., Chaksmithanont, P., Panomsuk, S., Nattapulwat, N., Plianwong, S., Patrojanasophon, P., and Opanasopit, P., 2023. Fabrication of a novel high-density three-dimensional (3D)-printed device for domperidone tablets. Thai Bulletin of Pharmaceutical Sciences. 18: 97-105.
Charoenying, T., Patrojanasophon, P., Ngawhirunpat, T., Rojanarata, T., Akkaramongkolporn, P., and Opanasopit, P., 2020a. Fabrication of floating capsule-in- 3D-printed devices as gastro-retentive delivery systems of amoxicillin. Journal of Drug Delivery Science and Technology. 55: 101393.
Charoenying, T., Patrojanasophon, P., Ngawhirunpat, T., Rojanarata, T., Akkaramongkolporn, P., and Opanasopit, P., 2020b. Three-dimensional (3D)-printed devices composed of hydrophilic cap and hydrophobic body for improving buoyancy and gastric retention of domperidone tablets. European Journal of Pharmaceutical Sciences. 155: 105555.
Charoenying, T., Opanasopit, P., Ngawhirunpat, T., Rojanarata, T., Akkaramongkolporn, P., and Patrojanasophon, P., 2023. Development of a novel tablet-shaped floating 3D-printed device with adjustable floating time as floating drug delivery systems provided zero-order release kinetics. Journal of Drug Delivery Science and Technology. 84: 104506.
Fafenrot, S., Grimmelsmann, N., Wortmann, M., and Ehrmann, A., 2017. Three-dimensional (3D) printing of polymer-metal hybrid materials by fused deposition modeling. Materials. 10: 1199.
Fu, J., Yin, H., Yu, X., Xie, C., Jiang, H., Jin, Y., and Sheng, F., 2018. Combination of 3D printing technologies and compressed tablets for preparation of riboflavin floating tablet-in-device (TiD) systems. International Journal of Pharmaceutics. 549: 370-379.
Goyanes, A., Buanz, A.B., Basit, A.W., and Gaisford, S., 2014. Fused-filament 3D printing (3DP) for fabrication of tablets. International Journal of Pharmaceutics. 476: 88-92.
Hattori, Y., Haruna, Y., and Otsuka, M., 2012. Dissolution process analysis using model-free Noyes-Whitney integral equation. Colloids and Surfaces. B, Biointerfaces. 102: 227-231.
Harikumar, S.L. and Sharma, A., 2012. Development and evaluation of bromhexine hydrochloride floating microparticulates. Asian Journal of Pharmaceutics. 6: 38-43.
Huanbutta, K., and Sangnim, T. 2019. Design and development of zero-order drug release gastroretentive floating tablets fabricated by 3D printing technology. Journal of Drug Delivery Science and Technology. 52: 831-837.
Huanbutta, K., Sriamornsak, P., Kittanaphon, T., Suwanpitak, K., Klinkesorn, N., and Sangnim, T. 2021. Development of a zero-order kinetics drug release floating tablet with anti–flip-up design fabricated by 3D-printing technique. Journal of Pharmaceutical Investigation. 51: 213-222.
Mandal, U.K., Chatterjee, B., and Senjoti, F.G., 2016. Gastro-retentive drug delivery systems and their in vivo success: A recent update. Asian Journal of Pharmaceutical Sciences. 11: 575-584.
Nayak, A., Malakar, J., and Sen, K., 2010. Gastroretentive drug delivery technologies: Current approaches and future potential. Indian Journal of Pharmaceutical Education and Research. 1: 1-12.
Pawar, V.K., Kansal, S., Garg, G., Awasthi, R., Singodia, D., and Kulkarni, G.T., 2011. Gastroretentive dosage forms: a review with special emphasis on floating drug delivery systems. Drug Delivery. 18: 97-110.
Qi, X., Ren, Y., and Wang, X., 2017. New advances in the biodegradation of poly(lactic) acid. International Biodeterioration and Biodegradation. 117: 215-223.
Redwood, B., Schoffer, F., and Garret, B., 2017. Coers & Roest, Amsterdam, The Netherlands.
Shin, S., Kim, T.H., Jeong, S.W., Chung, S.E., Lee, D.Y., Kim, D.H., and Shin, B.S., 2019. Development of a gastroretentive delivery system for acyclovir by 3D printing technology and its in vivo pharmacokinetic evaluation in Beagle dogs. PLoS One. 14: e0216875.
Song, J.H., Murphy, R.J., Narayan, R., and Davies, G.B., 2009. Biodegradable and compostable alternatives to conventional plastics. Philosophical Transactions of the Royal Society B. 364: 2127-2139.
Srikanth Meka, V., Ee Li, C., and Sheshala, R., 2016. Design and statistical optimization of an effervescent floating drug delivery system of theophylline using response surface methodology. Acta Pharmaceutica. 66: 35-51.
Tripathi, J., Thapa, P., Maharjan, R., and Jeong, S.H., 2019. Current state and future perspectives on gastroretentive drug delivery systems. Pharmaceutics. 11: 193.
Vaz, V.M. and Kumar, L., 2021. 3D printing as a promising tool in personalized medicine. AAPS PharmSciTech. 22: 49.
OPEN access freely available online
Natural and Life Sciences Communications
Chiang Mai University, Thailand. https://cmuj.cmu.ac.th
Thapakorn Charoenying1, Prasert Akkaramongkolporn1, Praneet Opanasopit1, Prasopchai Patrojanasophon1 and Supusson Pengnam2,*
1 Pharmaceutical Development of Green Innovations Group (PDGIG), Department of Industrial Pharmacy, Faculty of Pharmacy, Silpakorn University, Nakhon Pathom 73000, Thailand.
2 Pharmaceutical Development of Green Innovations Group (PDGIG), Department of Biomedicine and Health Informatics, Faculty of Pharmacy, Silpakorn University, Nakhon Pathom 73000, Thailand.
Corresponding author: Supusson Pengnam E-mail: pengnam_s@su.ac.th
Total Article Views
Editor: Nisit Kittipongpatana
Chiang Mai University, Thailand
Article history:
Received: April 16, 2023;
Revised: August 10, 2023;
Accepted: August 21, 2023;
Online First: August 25, 2023

