
Effects of Antidesma thwaitesianum Müll. Arg. Fruit Extract on Radical Scavenging and Apoptotic Properties
Supaporn Khamchun, Natthapak Sillawatthumrong, Wimonrut Insuan, Wipob Suttana, Daoyot Daorueang, Plubplung Sansai and Orapin Insuan*Published Date : August 23, 2023
DOI : https://doi.org/10.12982/NLSC.2023.062
Journal Issues : Number 4, October-December 2023
Abstract Antidesma thwaitesianum Müll. Arg. is one of a tropical fruit which is a good source of phenolic compounds. Here, we investigated the in vitro radical scavenging activity and effect of chlorogenic acid (CGA) and A. thwaitesianum fruit extract (AFE) against H2O2-induced cytotoxicity in RAW264.7 macrophage cells. Chemical components in the extract were analyzed by colorimetric method and high performance liquid chromatography. The results exhibited that the amount of total phenolic and total flavonoid contents in AFE were 25.486 ± 0.370 mg of gallic acid equivalents (GAE)/g extract and 7.264 ± 0.312 mg of catechin equivalents (CE)/g extract, respectively, while 114 μg/g crude extract CGA was detected in AFE. AFE showed scavenging activity against ABTS and DPPH radicals, with a 50% inhibition concentration of 11.435 ± 0.381 and 2.063 ± 0.022 mg/mL, respectively. Pretreatment with both CGA (10 µg/mL) and AFE (400 µg/mL) significantly restored cell viability in H2O2-induced cytotoxicity in RAW264.7 macrophage cells, and only CGA at the concentrations of 5 and 10 µg/mL could protect cells from apoptosis. In conclusion, CGA-containing AFE has potent in vitro antioxidant activity and can increase the cell survival in H2O2-induced RAW264.7 macrophage cells. These results support the nutritional and medical value of A. thwaitesianum fruit for health-promoting benefits.
Keywords: Antidesma thwaitesianum Müll. Arg., Chlorogenic acid, Antioxidant activity, Anti-apoptotic activity, RAW264.7 macrophage cells
Funding: This work was supported by the Thailand Science Research and Innovation Fund and the University of Phayao (Grant No. FF65-RIM139).
Citation: Khamchun, S., Sillawatthumrong, N., Insuan, W., Suttana,W., Daorueang, D., Sansai, P., and Insuan, O. 2023. Effects of Antidesma thwaitesianum Müll. Arg. fruit extract on radical scavenging and apoptotic properties. Natural and Life Sciences Communications. 22(4): e2023062.
INTRODUCTION
Phenolic compound-rich fruits are good sources for health-promoting benefits in functional foods and nutraceuticals (Yingngam et al., 2016). The major classes of phenolic compounds found in fruits are phenolic acids, flavonoids, stilbenes, lignans, and tannins (proanthocyanidins) (Rupasinghe et al., 2014). Phenolic acids and flavonoids are the most abundant phenolic compounds in the diet (60% and 30% of total dietary phenolic compounds, respectively)(Rupasinghe et al., 2014). Hydroxycinnamic acids and hydroxybenzoic acids are two main classes of phenolic acids, whereas flavonoids can be divided into six groups depending on their chemical structure, i.e. flavanones, flavones, flavonols, flavan-3-ols, anthocyanins, and dihydrochalcones (Rupasinghe et al., 2014; Jorjong et al., 2015; Laura et al., 2019). Fruits containing phenolic acids (caffeic, chlorogenic, p-coumaric, ferulic, gallic, and ellagic acids), flavonols (kaempferol, quercetin, and myricetin), and anthocyanins (pelargonidin, cyanidin, peonidin, petunidin, malvidin, and delphinidin) exhibit beneficial effects such as antioxidant, antibacterial, anti-inflammatory, and anticancer properties (Nohynek et al., 2006; Koponen et al., 2007; Seeram, 2008; Rodrigues et al., 2011; Rupasinghe et al., 2014; Jorjong et al., 2015).
Tropical fruit is one of the interesting resources for phenolic compounds. Antidesma thwaitesianum Müll. Arg. or mamao is a plant classified in the family Euphorbiaceae (Hansakul et al., 2015). It is widely cultivated in North-Eastern and Northern areas of Thailand (Kemsawasd and Chaikham, 2021). Its ripe fruit is purplish red to dark purple (Hoffmann, 1999; Hansakul et al., 2015). Its products are commonly consumed, particularly as juice drinks, red wine, jam, and healthy foods (Chaikham et al., 2016; Butkhup and Samappito, 2018; Kwaw et al; 2018). Ripe fruits of A. thwaitesianum have several biological properties, including antioxidant, anticancer, antimicrobial, anti-apoptotic, anti-inflammatory, and hypoglycemic effects (Puangpronpitag et al., 2008; Puangpronpitag et al., 2011; Hassakul et al., 2015; Anantachoke et al., 2016; Pheungsomphane et al., 2019; Udomkasemsab et al., 2019; Thongchuai et al., 2022). Large amounts of bioactive phenolic compounds have been found in mamao cultivars, including phenolic acids (gallic acid, vanillic acid, chlorogenic acid, caffeic acid, ferulic acid, sinapinic acid, and cinnamic acid), flavonoids (quercetin, myricetin, (+)-catechin, and (−)-epicatechin), and anthocyanins, especially cyanidin-3-O-glucoside, cyanidin-3-rutinoside, and malvidin (Jorjong et al., 2015; Krongyut and Sutthanut, 2019; Natewong et al., 2022). A previous study showed that A. thwaitesianum fruit extract exhibits cellular antioxidant activity and cytotoxicity against human breast MCF7 and human lung large cell carcinoma COR-L23 cells (Hansakul et al., 2015). This fruit extract significantly reduces the production levels of pro-inflammatory cytokines and mediators, including nitric oxide (NO), interleukin (IL)-1β, and tumor necrosis factor (TNF)-α in RAW264.7 macrophage cells (Thongchuai et al., 2022). It also remarkably decreases the expression of pro-inflammatory genes such as TNF-α, IL-6, and endothelial nitric oxide synthase (eNOS)(Udomkasemsab et al., 2018). It has been reported that the fruit extract of A. thwaitesianum containing phenolic compounds including cyanidin, ferulic acid, caffeic acid, vanillic acid, and protocatechuic acid has a photoprotective effect against UVB-induced keratinocyte cell damage by inhibiting oxidative stress and inflammation (Natewong et al., 2022). Moreover, A. thwaitesianum seed and marc extracts exhibited the protective effect against hydrogen peroxide (H2O2)-induced apoptosis in human breast epithelial (MCF10A) cells by inhibiting poly [ADP-ribose] polymerase (PARP) and caspase-3 cleavage, inducing the expression of anti-apoptotic Bcl-2, and down-regulating pro-apoptotic Bax (Puangpronpitag et al., 2011). To provide data to support product development and applications of A. thwaitesianum fruit for health promotion, this work aims to determine the radical scavenging activity of A. thwaitesianum fruit extract in vitro and investigate the effect of the extract on cell apoptosis in RAW264.7 macrophages.
MATERIALS AND METHODS
Chemicals and reagents
Folin–Ciocalteu reagent was obtained from Merck (Darmstadt, Germany). 2,2-diphenyl-1-picrylhydrazyl (DPPH), 2,2´-Azino-bis (3-ethylbenzthiazoline-6-sulfonic acid)(ABTS) diammonium salt, (+)-catechin hydrate, and butylated hydroxytoluene (BHT) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Chlorogenic acid hydrate (> 98.0% purity) was obtained from Tokyo Chemistry Industry (Chuo-ku, Tokyo, Japan). Thiazolyl blue tetrazolium bromide (MTT) was purchased from AppliChem GmbH (Darmstadt, Germany). Dulbecco’s Modified Eagle’s Medium (DMEM), fetal bovine serum, streptomycin, and penicillin were obtained from Invitrogen Gibco (Grand Island, NY, USA). All other chemicals were analytical grade.
Plant extraction
Ripe fruits of A. thwaitesianum were collected from Phayao Province, Thailand at 19°05´29”N, 99°48´33”E; 515 m altitude during December 2020. The voucher specimen (QBG No. 128865) was deposited at Queen Sirikit Botanic Garden Herbarium, Chiang Mai, Thailand. Fruits were dried using a hot air oven at 50 °C and then ground into powder. A 300 g sample of A. thwaitesianum dried ripe fruit powder was mixed with 900 mL of 50% v/v ethanol, stirred at 50 °C for 45 min and then filtered through Whatman No.1 filter paper. The filtrate was evaporated under reduced pressure at 40 °C using a rotary evaporator connected to a water bath (EYELA, Tokyo Rikakikai Co., Ltd., Tokyo, Japan) and lyophilized using a freeze dryer (Labconco Corporation, MO, USA). The A. thwaitesianum fruit extract (AFE) was stored at −20 °C.
Total phenolic content assay
The total phenolic content (TPC) was determined according to the Folin–Ciocalteu method as described by Inboot et al. (2012) with some modifications. Diluted AFE (10 μL) was reacted with 50 µL of a mixture of Folin–Ciocalteu reagent and distilled water. After incubation at room temperature for 10 min, 100 µL of 7% Na2CO3 and 80 µL of distilled water were added and the mixture incubated at 45 °C for 15 min. The visible absorbance at 765 nm was measured after maintaining the sample at room temperature for 5 min. The TPC was calculated and expressed as milligrams of gallic acid equivalents (GAE) per gram of extract (mg GAE/g extract)(Inboot et al., 2012).
Total flavonoid content assay
The total flavonoid content (TFC) was determined according to the aluminum chloride colorimetric method as reported previously (Inboot et al., 2012). AFE (30 μL) was mixed with 177 µL of the reagent mixture containing distilled water, 5% NaNO2, and 10% AlCl3.6H2O and incubated at room temperature for 20 min. Then, 60 µL of 1 M NaOH and distilled water were added, and the mixture kept for 5 min at room temperature. The visible absorbance at 532 nm was determined. The TFC was calculated by calibration curves using catechin as a standard and expressed as milligrams of catechin equivalents (CE) per gram of extract (mg CE/g extract).
HPLC analysis
The chlorogenic acid (CGA) present in AFE was analyzed by HPLC (Waters e2695, separation module, Waters, USA) in gradient mode. All analyses were performed with the temperature maintained at 40 °C on an analytical C18 column (250 mm × 4.6 mm, 3 μm) (YMC-Triart C18, Japan). The injection volume and flow rate were 10 μL and 0.8 mL/min, respectively. Gradient elution was performed with mobile phases A (0.1% formic acid) and B (acetonitrile) as follows: 0–1.5 min, 10% B; 2 min, 17% B; 3 min, 20% B; 5 min, 24% B; 10 min, 35% B; and 12–20 min, 50% B. The detection wavelength was monitored at 254 nm. The peak was identified by comparison of retention times with the CGA standard and the spectra as well as by the spiked of standard. Quantification of CGA was calculated using external standard curve.
ABTS radical scavenging activity
The radical cation ABTS scavenging activity of AFE or CGA was determined according to Re et al. (1999). with some modifications. To generate ABTS radicals, 7 mM ABTS and 2.45 mM potassium persulfate were mixed and incubated in the dark at room temperature for 12–16 h. The ABTS stock solution was diluted with distilled water to an absorbance of 0.70 ± 0.02 at 734 nm. Then, 20 μL of the extract was mixed with 2000 μL of the ABTS solution, and the absorbance at 734 nm was measured after incubation in the dark at room temperature for 6 min (Re et al., 1999). The percentage of ABTS scavenging activity was calculated using the equation: ABTS scavenging activity (%) = [(Acontrol − Asample)/Acontrol] × 100 where A indicates the absorbance. The inhibition concentration of the extract that reduced 50% of ABTS radical formation (IC50) was calculated using a calibration curve in the linear range by plotting the concentration of the sample vs. the percentage of radical scavenging activity.
DPPH radical scavenging activity
The DPPH radical scavenging activity of AFE and CGA was determined according to the method of Phongthai et al. (2018) with some modification. Fifty microliters of the extract were added into 200 µL of 0.1 mM DPPH solution in ethanol. The absorbance of the reaction mixture was determined at 517 nm after incubation in the dark at room temperature for 30 min. The percentage of DPPH scavenging activity was calculated and IC50 was determined (Phongthai et al., 2018).
Cell viability assay
The RAW264.7 mouse macrophage cell line was purchased from the American Type Culture Collection (ATCC® number TIB-71) and cultured in DMEM supplemented with 10% fetal bovine serum and 100 units/mL penicillin–streptomycin under a humidified (95%) atmosphere of 5% CO2 at 37 °C. The cytotoxicity of the extract or H2O2 to RAW264.7 macrophage cells was determined by performing the MTT assay. The cells were plated in a 96-well plate at a density of 2 x 104 cells/well (cell viability > 95%) for 24 h. Then the cells were treated with various concentrations of the extract or H2O2 for another 24 h or pre-incubated with different concentrations of the extract for 20 h before being exposed to 900 μM H2O2 for 4 h. MTT solution (final concentration of 0.5 mg/mL) was added to each well and the plate incubated at 37 °C for 4 h. The supernatant was removed, formazan crystals were dissolved in DMSO, and the absorbance was determined at 540 nm using a microplate reader (Berthold Technologies GmbH & Co. KG, Bad Wildbad, Germany). Cell viability was calculated as the percentage of survival cells in treated wells compared to control wells.
Annexin V-APC/PI apoptosis assay
The amount of apoptosis was determined with a flow cytometer using annexin V-allophycocyanin (APC)/propidium iodide (PI) double staining. Briefly, RAW264.7 macrophage cells were pretreated with the extract for 20 h and were then exposed to H2O2 to a final concentration of 900 μM for 4 h. Cells were collected by centrifugation and washed twice with PBS. The cells were resuspended in the binding buffer and stained with APC-conjugated annexin V and PI (BioLegend, San Diego, CA, USA) in the dark at room temperature for 20 min. The fluorescence intensity of the cells was analyzed using a flow cytometer (Beckman Coulter, Indianapolis, IN, USA). A schematic plot was used to exhibit the results, in which the lower left quadrant represents live cells, the upper left quadrant represents necrotic cells, and the lower right and upper right quadrants represent early and late apoptotic cells, respectively.
Statistical analysis
All results are presented as the mean ± standard deviation (SD). One-way analysis of variance (ANOVA) with P < 0.05 was used to compare the differences between the treatments.
RESULTS
Phytochemicals
The TPC and TFC of AFE were 25.468 ± 0.370 mg GAE/g extract and 7.264 ± 0.312 mg CE/g extract, respectively. To identify the CGA content, the AFE was prepared and compared with CGA standard using the HPLC method. The standard solution was injected to confirm the retention time. The chromatograms are shown in Figure 1(A, B) corresponding to a CGA standard and the spiked solution of AFE, respectively, with the CGA eluted at 7.41 min. Sharp and symmetrical peaks were obtained for CGA when analyzed under separation conditions. The HPLC profile of AFE in Figure 1(B) shows that the fruit extract contains many chemical components. The AFE had four peaks with one of CGA at 7.41 min and other three peaks at 4.0, 8.7 and 9.3 min were not identified in this study. As reported by other researchers compared the elution profile and results from reversed phase HPLC analysis, the first peak at 4.0 min may be a small molecular organic acid or high polarity component than CGA that contains in AFE such as gallic acid. The two strong peaks at 8.7 and 9.3 min that may be a large molecular organic acid or the component have closed polarity to CGA such as caffeic acid, vanillic acid, catechin or epicatechin (Jorjong et al., 2015; Poontawee et al., 2015). The linear regression equation for the CGA was y = 21711x − 3426.2, with a correlation coefficient of 0.999. The results from the linear regression analysis of the data for CGA in the AFE show a concentration of 114 µg/g crude extract.
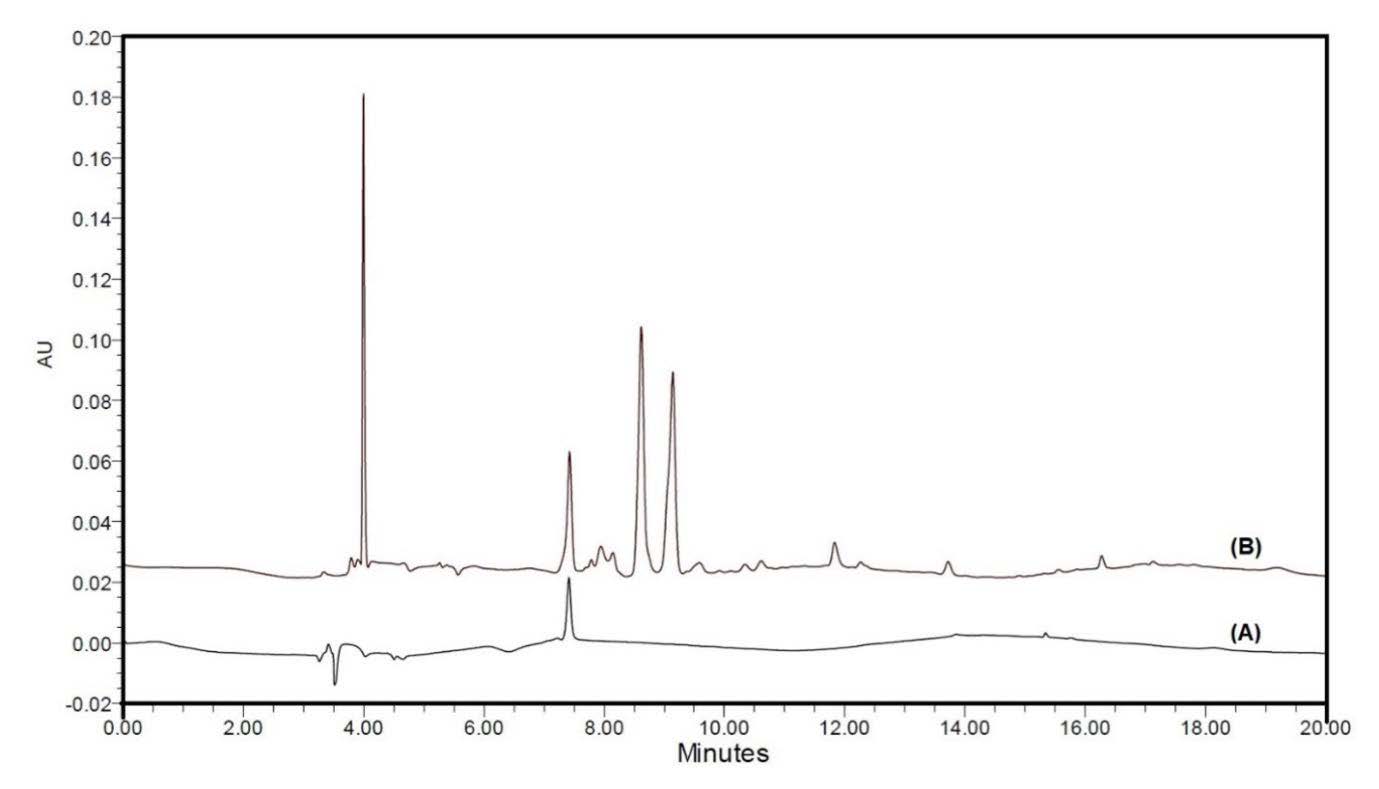
Figure 1. Chromatography profiles of chlorogenic acid (CGA) standard (A) and A. thwaitesianum fruit extract (AFE) spiked with CGA standard (B) at 254 nm.
Radical scavenging activity of chlorogenic acid and A. thwaitesianum fruit extract
The antioxidant properties of CGA and AFE were determined using ABTS cation and DPPH radical scavenging assays. The scavenging activity of AFE against ABTS and DPPH radicals, represented by the IC50, was 11.435 ± 0.381 and 2.063 ± 0.022 mg/mL, respectively. On the other hand, CGA had stronger antioxidant properties than AFE, with an IC50 of ABTS and DPPH radicals of 0.521 ± 0.001 and 0.049 ± 0.001 mg/mL, respectively (Table 1). The positive antioxidant BHT showed radical scavenging potential against ABTS and DPPH radicals, with an IC50 of 0.669 ± 0.015 and 0.418 ± 0.006 mg/mL, respectively. The ABTS and DPPH radical scavenging effects of BHT were stronger than AFE. Additionally, CGA showed high potential antioxidant activity on scavenging of DPPH radicals compared to BHT, whereas there was no significant difference on ABTS radical scavenging.
Table 1. Antioxidant activity of chlorogenic acid and A. thwaitesianum fruit extract.
|
Samples |
Radical scavenging activities (IC50, mg/mL) |
|
|
ABTS |
DPPH |
|
|
AFE |
11.435 ± 0.381a,b |
2.063 ± 0.022a,b |
|
CGA |
0.521 ± 0.001c |
0.049 ± 0.001a,c |
|
BHT |
0.669 ± 0.015 |
0.418 ± 0.006 |
Note: Data are presented as the mean ± SD (n = 3). a P <0.05 indicated significant differences from BHT positive control, b,c P < 0.05 within a column indicate significant differences for AFE and CGA. AFE: A. thwaitesianum fruit extract, CGA: chlorogenic acid, BHT: butylated hydroxytoluene
Cytotoxic effects of chlorogenic acid and A. thwaitesianum fruit extract in RAW264.7 macrophage cells
To investigate the cytotoxicity of CGA and AFE in RAW264.7 macrophage cells, the cells were treated with various concentrations of CGA (1–100 µg/mL) or AFE (10–400 µg/mL) for 24 h. Cell viability was determined by MTT assay. As shown in Figure 2A and 2B, neither CGA nor AFE had a toxic effect on RAW264.7 macrophage cells (cell viability ≥ 80%) and the treatment of 10% DMSO (toxic control) reduced RAW264.7 cell viability to 12.97 ± 0.18 % (data not shown). Therefore, 1, 5, and 10 µg/mL CGA and 100, 200, and 400 µg/mL AFE were used as the suitable concentrations for the subsequent experiments.
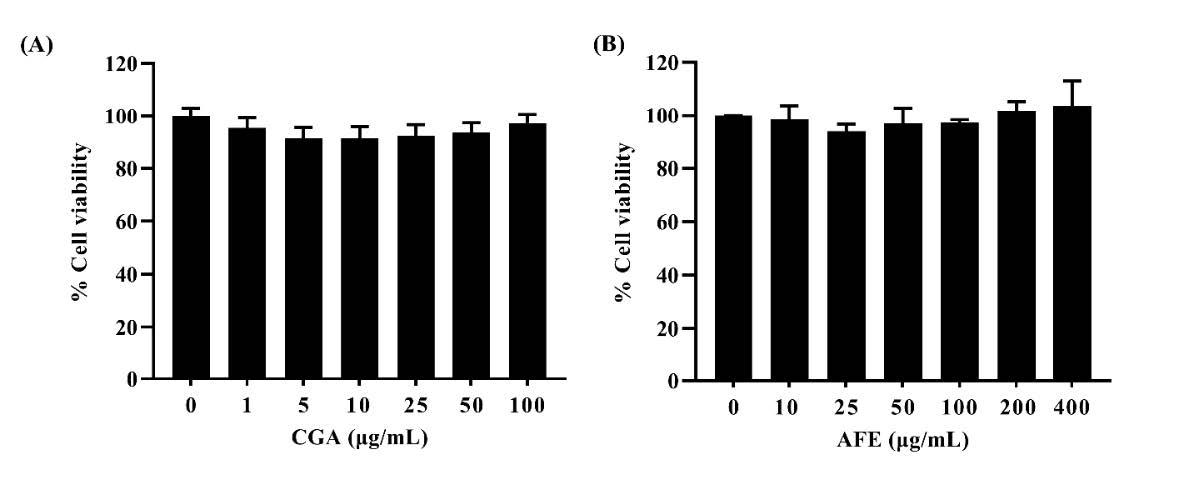
Figure 2. Cytotoxicity of chlorogenic acid and A. thwaitesianum fruit extract in RAW264.7 macrophage cells. (A) Cells were treated with various concentrations of CGA (1–100 µg/mL) and (B) Cells were treated with various concentrations of AFE (10–400 µg/mL). Data are presented as the mean ± SD of three independent experiments. AFE: A. thwaitesianum fruit extract, CGA: chlorogenic acid.
Effects of chlorogenic acid and A. thwaitesianum fruit extract on H2O2-induced cytotoxicity in RAW264.7 macrophage cells
To establish the experimental model, RAW264.7 macrophage cells were treated with different concentrations of H2O2 for 24 h, and the MTT assay was evaluated. Macrophage cells, one of the main targets of pro-oxidants, play an important role in detecting and eliminating microbial pathogens in the host defense system (Qin et al., 2019; Li et al., 2020). Hydrogen peroxide (H2O2) is a reactive oxygen species (ROS) that can rapidly penetrate cellular membranes and may damage cells through direct oxidation of biomolecules such as lipids, proteins, and DNA, or act as a signaling molecule to trigger intracellular pathways leading to cell death, hence it is normally used to investigate apoptosis or oxidative stress-induced cell injury in macrophage cells (Holmström and Finkel, 2014; Wang et al., 2018; Qin et al., 2019; Li et al., 2020). The results are presented in Figure 3; a significant decrease in cell viability was observed after RAW264.7 macrophage cells were treated with an increasing concentration of H2O2. When the concentration was greater than 400 µM, the cell viability was dramatically decreased (P < 0.05). The IC50 was determined to be 883.90 ± 96.87 µM (Figure 3A). At this point, 900 µM was selected for the subsequent experiments. To evaluate the protective effect of CGA and AFE against cytotoxicity induced by H2O2, RAW264.7 macrophage cells were treated with 1, 5, and 10 µg/mL CGA or 100, 200, and 400 µg/mL AFE for 20 h before treatment with 900 µM H2O2 for 4 h. As shown in Figure 3B and 3C, CGA and AFE reduced H2O2-induced cytotoxicity. In addition, pretreatment with CGA at the concentration of 10 µg/mL markedly restored the cell viability as compared with H2O2 treatment alone (P < 0.05), while pretreatment with AFE (400 µg/mL) could prevent this effect. The results revealed that CGA and AFE can protect cells against H2O2‑induced injury.
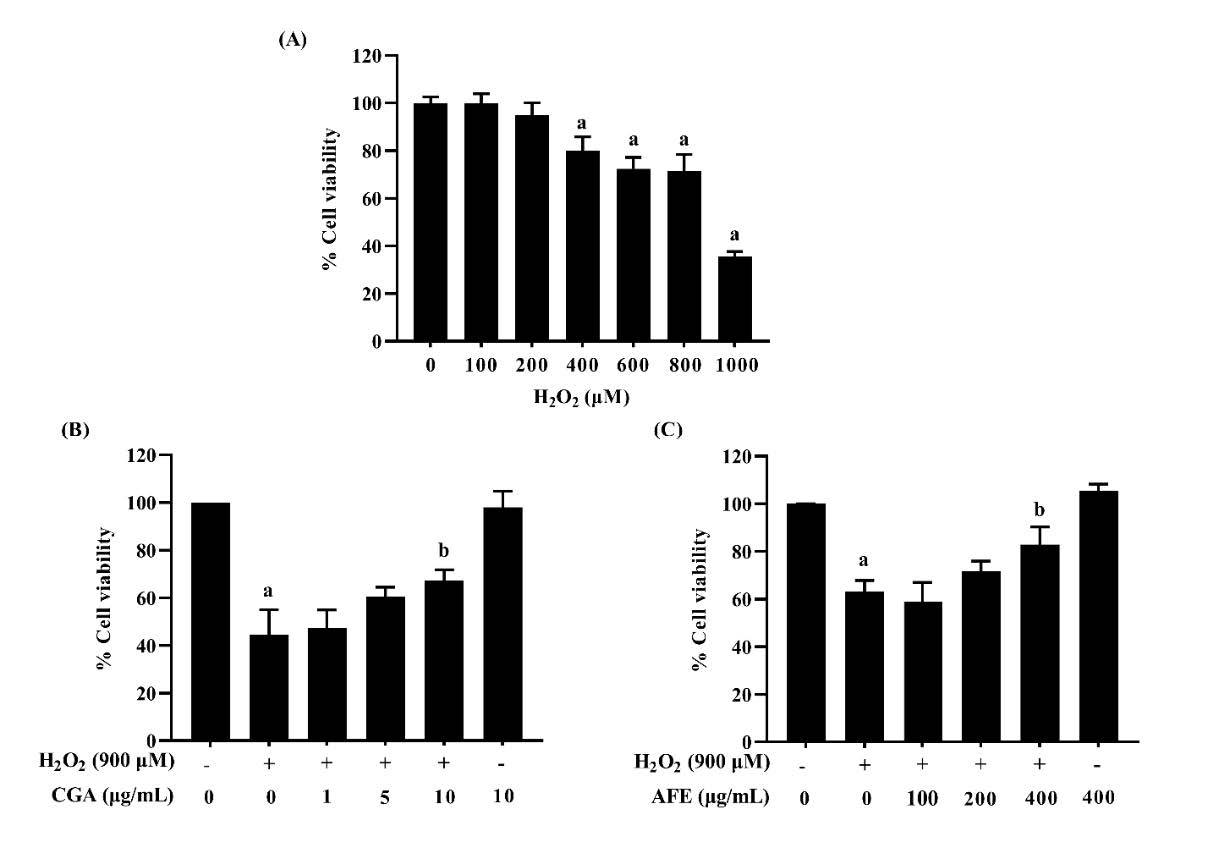
Figure 3. Protective effects of chlorogenic acid and A. thwaitesianum fruit extract against H2O2-induced cytotoxicity in RAW264.7 macrophage cells. (A) Cells were treated with various concentrations of H2O2 (100–1,000 µM), (B) Cells were treated with various concentrations of CGA for 20 h following 900 µM H2O2 for 4 h and (C) Cells were treated with various concentrations of AFE for 20 h following 900 µM H2O2 for 4 h. Data are presented as the mean ± SD of three independent experiments. aP < 0.05 compared with the control group, bP < 0.05 compared with the H2O2-treated group. AFE: A. thwaitesianum fruit extract, CGA: chlorogenic acid.
Effects of chlorogenic acid and A. thwaitesianum fruit extract on cell apoptosis in H2O2-induced RAW264.7 macrophage cells
To investigate whether the cytoprotective effect of CGA and AFE against H2O2 in RAW264.7 macrophage cells was associated with the inhibition of apoptosis, annexin V/PI double staining and flow cytometry analyses were performed in this study. The results exhibited that the ratio of early apoptosis (annexin V-positive and PI-negative cells) and late apoptosis (annexin V-positive and PI-positive cells) in the H2O2-treated group was markedly higher than that in the negative control group (P < 0.05). Pretreatment with CGA significantly decreased only the rate of early apoptosis in H2O2-treated cells (Figure 4A and 4B), while there were no significant differences among the AFE-treated groups (Figure 4C). On the other hand, the ratio of late apoptosis in CGA-treated groups and AFE-treated groups did not change. The results indicate that only CGA can prevent the transformation from the early to late stages of apoptosis.
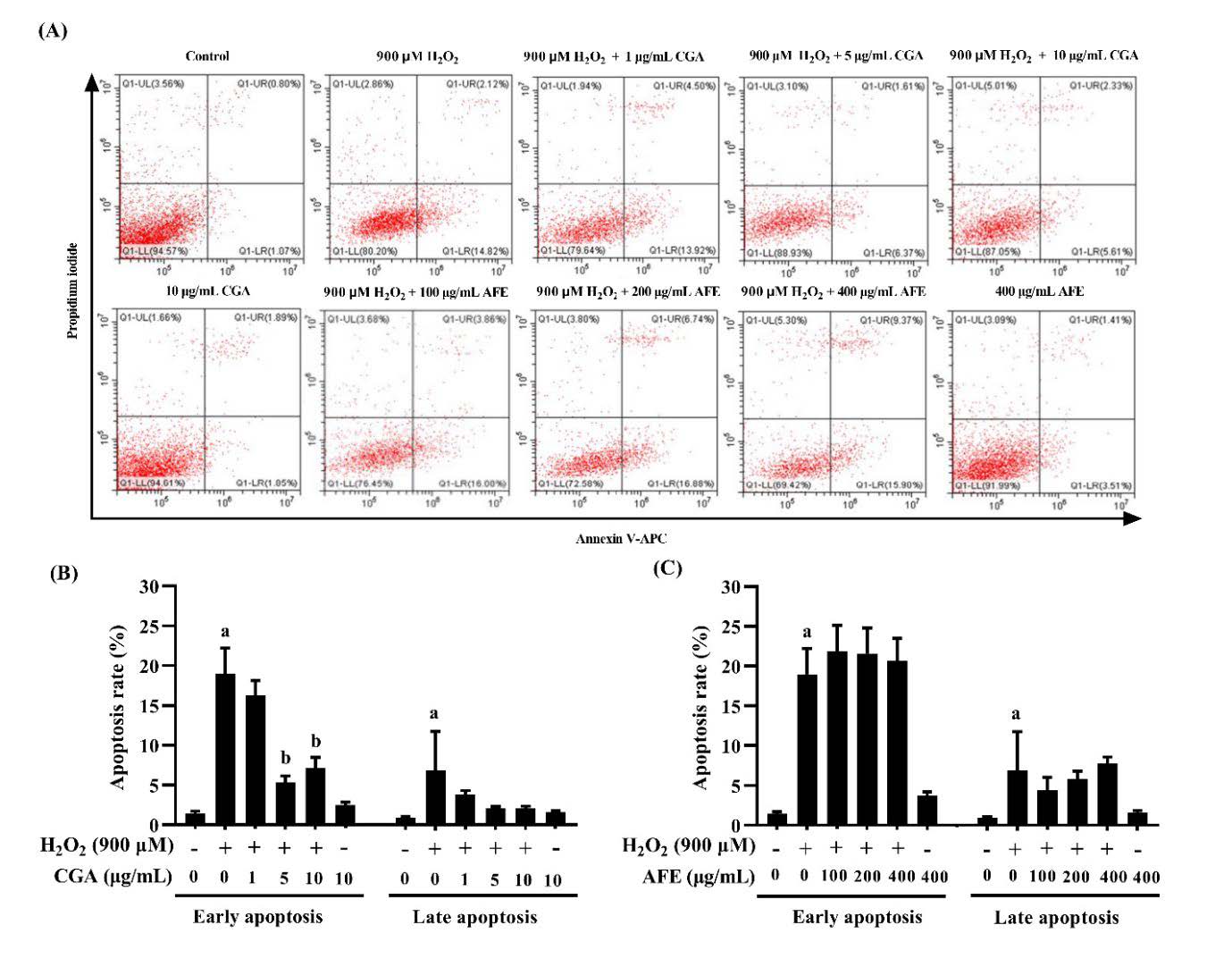
Figure 4. Effects of chlorogenic acid and A. thwaitesianum fruit extract on H2O2-induced apoptosis in RAW264.7 macrophage cells. (A) Cells were stained with allophycocyanin (APC)-conjugated annexin V and propidium iodide (PI) for flow cytometry analysis. The lower right quadrant represents early apoptosis (annexin V-positive and PI-negative cells), and the upper right quadrant represents late apoptosis (annexin V-positive and PI-positive cells)(B, C) percentages of apoptotic cells after treatment with CGA and AFE, respectively. Data are presented as the mean ± SD of three independent experiments, aP < 0.05 compared with the control group, bP < 0.05 compared with the H2O2-treated group. AFE: A. thwaitesianum fruit extract, CGA: chlorogenic acid.
DISCUSSION
ROS are normally generated by many redox processes in aerobic cellular metabolism (Valko et al., 2007). Overproduction of ROS can damage biological molecules, leading to cell death and tissue damage, and the development of chronic diseases, including nephritis, diabetes mellitus, cardiovascular diseases, and cancer (Alamed et al., 2009; Xu et al., 2012). However, the oxidation of biological molecules (e.g., lipids, proteins, and DNA) can be inhibited by antioxidants via inhibiting or eliminating the initiation or propagation of excess free radicals (Alamed et al., 2009). Nowadays, phenolic compounds are interesting components in functional foods and nutraceuticals because of their strong antioxidant properties (Poontawee et al., 2015). Interestingly, A. thwaitesianum (mamao in Thai) is one such fruit which is a good source of phenolic acids, flavonoids, and anthocyanins. Gallic acid, vanillic acid, CGA, caffeic acid, ferulic acid, sinapinic acid, and cinnamic acid are the major phenolic acids in ripe fruits (Jorjong et al., 2015). The most abundant flavonoids detected are quercetin, myricetin, (+)-catechin, and (−)-epicatechin, and anthocyanins, mainly cyanidin-3-O-glucoside, cyanidin-3-rutinoside, and malvidin (Jorjong et al., 2015). The fruit of A. thwaitesianum possesses free radical scavenging potential due to the presence of phenolic compounds, including gallic acid, ferulic acid, caffeic acid, vanillic acid, protocatechuic acid, and anthocyanins (Jorjong et al., 2015; Krongyot et al., 2019; Natewong et al., 2022). Phenolic compounds exert a potent antioxidant activity by scavenging free radicals via the electron and proton transfer on hydroxyl groups that stabilize free radicals (Natewong et al., 2022). In this study, we have evaluated the antioxidant potential and apoptotic effect of AFE and CGA, one of the most available phenolic acids in A. thwaitesianum fruit. CGA is a group of secondary phenolic metabolites that has been found in many plants including coffee, tea, and berry fruits (Naveed et al., 2018). It is considered a well-known antioxidant, anti-inflammatory, and anticancer agent (Naveed et al., 2018; Han et al., 2019). It has been reported that CGA has antioxidant activity by decreasing the generation of ROS and protecting against H2O2-induced apoptosis in MC3T3-E1 osteoblast cells (Han et al., 2017). The present study indicates that the AFE contained phenolic acids and flavonoids. Our HPLC data demonstrated the presence of CGA in AFE (Figure 1). In correlation with previous studies, we found that CGA-containing AFE has scavenging activity against ABTS and DPPH radicals. The free radical scavenging activity of AFE might be correlated with the presence of its chemical constituents (Hansakul et al., 2015; Poontawee et al., 2015; Natewong et al., 2022). However, the antioxidant properties of AFE were less than CGA and positive antioxidant BHT.
Increased ROS accumulation due to oxidative stress has been recognized as one of the mechanisms leading to apoptosis related to mitochondrial injury (Kwon et al., 2019). During the induction of ROS-mediated apoptosis, excessive ROS cause free radical damage to cell membrane phospholipids, which leads to mitochondrial membrane depolarization, and results in the loss of mitochondrial membrane potential (MMP)(Kwon et al., 2019). Subsequently, cytochrome c is released and activate the caspase cascade for the initiation of the intrinsic apoptosis pathway (Kwon et al., 2019). Although apoptosis is important for normal development and homeostasis, and necessary for removing damaged, infected, or neoplastic cells, too much apoptotic cell death can lead to the development of many diseases such as Alzheimer’s, Parkinson’s, and AIDS (Kannan and Jain, 2000). A previous study revealed that activation of caspases and a decrease in the Bcl-2/Bax ratio have a critical role in H2O2-induced apoptosis in RAW264.7 macrophage cells (Kwon et al., 2019). Excessive ROS are converted to H2O2, which easily infiltrate cell membranes and produce highly reactive and toxic hydroxyl radicals (•OH) through the Fenton reaction, finally triggering macrophage death (Lin et al., 2019). Currently, the protective effects of numerous natural antioxidants against H2O2-mediated cytotoxicity have been reported (Han et al., 2017; Kwon et al., 2019; Lin et al., 2019). The present study demonstrated that pretreatment with CGA might improve cellular stress response in H2O2-induced injury in RAW264.7 macrophage cells, resulting in cell viability recovery and reduced apoptosis (Figure. 3B and 4B). Additionally, AFE can increase cell survival, while it does not attenuate apoptosis. This may be due to the presence of many compounds in this crude AFE, which exert their effect.
CONCLUSION
Our finding confirms that CGA-containing AFE has potent in vitro antioxidant properties and can improve survival of H2O2-induced RAW264.7 macrophage cells. For safe usage and to provide supporting data in product development and applications of this fruit extract, further studies on the cellular protective potential and molecular mechanisms of AFE containing CGA should be undertaken in an animal model.
ACKNOWLEDGMENTS
The authors are very thankful to the Department of Medical Technology, School of Allied Health Sciences, University of Phayao for providing instruments.
AUTHOR CONTRIBUTIONS
Supaporn Khamchun performed the experiment, analyzed the data, and wrote the original draft manuscript related to apoptotic property. Natthapak Sillawatthumrong, Wipob Suttana, Daoyot Daorueang, and Plubplung Sansai performed the experiments and analyzed the data related to antioxidant and apoptotic properties. Wimonrut Insuan performed the experiment, analyzed the data, and wrote the original draft manuscript related to phytochemical analysis. Orapin Insuan designed and performed the experiments, analyzed the data, and wrote original draft and edited the manuscript. All co-authors revised and approved the final version of the manuscript.
CONFLICT OF INTEREST
No potential conflict of interest is reported by the authors.
REFERENCES
Alamed, J., Chaiyasit, W., McClements, D.J., and Decker, E.A. 2009. Relationships between free radical scavenging and antioxidant activity in foods. Journal of Agricultural and Food Chemistry. 57(7): 2969–2976.
Anantachoke, N., Lomarat, P., Praserttirachai, W., Khammanit, R., and Mangmool, S. 2016. Thai fruits exhibit antioxidant activity and induction of antioxidant enzymes in HEK-293 cells. Evidence-Based Complementary and Alternative Medicine. 2016: 6083136.
Butkhup, L., and Samappito, S. 2018. An analysis on flavonoids contents in mao luang fruits of fifteen cultivars (Antidesma bunius), grown in Northeast Thailand. Pakistan Journal of Biological Sciences. 11(7): 996–1002.
Chaikham, P., Prangthip, P., and Seesuriyachan, P. 2016. Ultra-sonication effects on quality attributes of maoberry (Antidesma bunius L.) juice. Food Science and Technology Research. 22(5): 647–654.
Han, D., Chen, W., Gu, X., Shan, R., Zou, J., Liu, G., et al. 2017. Cytoprotective effect of chlorogenic acid against hydrogen peroxide-induced oxidative stress in MC3T3-E1 cells through PI3K/Akt-mediated Nrf2/HO-1 signaling pathway. Oncotarget. 8(9): 14680–14692.
Han, D., Gu, X., Gao, J., Wang, Z., Liu, G., Barkema, H.W., et al. 2019. Chlorogenic acid promotes the Nrf2/HO-1 anti-oxidative pathway by activating p21Waf1/Cip1 to resist dexamethasone-induced apoptosis in osteoblastic cells. Free Radical Biology and Medicine. 137: 1–12.
Hansakul, P., Dechayont, B., Phuaklee, P., Prajuabjinda, O., Juckmeta, T., and Itharat, A. 2015. Cytotoxic and antioxidant activities of Antidesma thwaitesianum Müll Arg (Euphorbiaceae) fruit and fruit waste extracts. Tropical Journal of Pharmaceutical Research. 14(4): 627–634.
Hoffmann, P. 1999. The genus Antidesma (Euphorbiaceae) in Madagascar and the Comoro islands. Kew Bulletin. 54: 877–885.
Holmström, K.M., and Finkel, T. 2014. Cellular mechanisms and physiological consequences of redox-dependent signalling. Nature Reviews Molecular Cell Biology. 15(6): 411–421.
Inboot, W., Taya, S., Chailungka, A., Meepowpan, P., and Wongpoomchai, R. 2012. Genotoxicity and antigenotoxicity of the methanol extract of Cleistocalyx nervosum var. paniala seed using a Salmonella mutation assay and rat liver micronucleus tests. Molecular & Cellular Toxicology. 8: 19–24.
Jorjong, S., Butkhup, L., and Samappito, S. 2015. Phytochemicals and antioxidant capacities of Mao-Luang (Antidesma bunius L.) cultivars from Northeastern Thailand. Food Chemistry. 181: 248–255.
Kannan, K., and Jain, S.K. 2000. Oxidative stress and apoptosis. Pathophysiology. 7(3): 153–163.
Kemsawasd, V., and Chaikham, P. 2021. Alteration of bioactive compounds and antioxidative properties in thermal, ultra-high pressure and ultrasound treated maoberry (Antidesma bunius L.) juice during refrigerated storage. Current Research in Nutrition and Food Science Journal. 9(3): 904–916.
Koponen, J.M., Happonen, A.M., Mattila, P.H., and Törrönen, A.R. 2007. Contents of anthocyanins and ellagitannins in selected foods consumed in Finland. Journal of Agricultural and Food Chemistry. 55(4): 1612–1619.
Krongyut, O., and Sutthanut, K. 2019. Phenolic profile, antioxidant activity, and anti-obesogenic bioactivity of Mao Luang fruits (Antidesma bunius L.). Molecules. 24(22): 4109.
Kwaw, E., Ma, Y., Tchabo, W., Apaliya, M.T., Sackey, A.S., Wu, M., et al. 2018. Impact of ultrasonication and pulsed light treatments on phenolics concentration and antioxidant activities of lactic-acid-fermented mulberry juice. LWT – Food Science and Technology. 92: 61–66.
Kwon, D.H., Cha, H.J., Lee, H., Hong, S.H., Park, C., Park, S.H., et al. 2019. Protective effect of glutathione against oxidative stress-induced cytotoxicity in RAW 264.7 macrophages through activating the nuclear factor erythroid 2-related factor-2/heme oxygenase-1 pathway. Antioxidants. 8(4): 82.
Laura, A., Moreno-Escamilla, J.O., Rodrigo-García, J., and Alvarez-Parrilla, E. 2019. Phenolic compounds. In E.M. Yahia (Ed.). Postharvest physiology and biochemistry of fruits and vegetables (pp. 253–271). Elsevier, Amsterdam.
Li, J., Li, Y., Li, Y., Yang, Z., and Jin H. 2020. Physicochemical properties of collagen from Acaudina molpadioides and its protective effects against H2O2-induced injury in RAW264.7 cells. Marine Drugs. 18(7): 370.
Lin, X., Bai, D., Wei, Z., Zhang, Y., Huang, Y., Deng, H., et al. 2019. Curcumin attenuates oxidative stress in RAW264.7 cells by increasing the activity of antioxidant enzymes and activating the Nrf2-Keap1 pathway. PLoS One. 14(5): e0216711.
Natewong, S., Niwaspragrit, C., Ratanachamnong, P., Samatiwat, P., Namchaiw, P., and Jaisin, Y. 2022. Photo-protective and anti-inflammatory effects of Antidesma thwaitesianum Müll. Arg. fruit extract against UVB-induced keratinocyte cell damage. Molecules. 27(15): 5034.
Naveed, M., Hejazi, V., Abbas, M., Kamboh, A.A., Khan, G.J., Shumzaid, M., et al. 2018. Chlorogenic acid (CGA): A pharmacological review and call for further research. Biomedicine & Pharmacotherapy. 97: 67–74.
Nohynek, L.J., Alakomi, H.L., Kähkönen, M.P., Heinonen, M., Helander, I.M., Oksman-Caldentey, K.M., et al. 2006. Berry phenolics: Antimicrobial properties and mechanisms of action against severe human pathogens. Nutrition and Cancer. 54(1): 18–32.
Pheungsomphane, S., Tinchan, P., and Tayuan, C. 2019. Total phenolic content and biological activity of Antidesma thwaitesianum Müll. Arg. fruit extracts. Khon Kean Agriculture Journal. 47(1): 495–500.
Phongthai, S., D’Amico, S., Schoenlechner, R., Homthawornchoo, W., and Rawdkuen, S. 2018. Fractionation and antioxidant properties of rice bran protein hydrolysates stimulated by in vitro gastrointestinal digestion. Food Chemistry. 240: 156–164.
Poontawee, W., Natakankitkul, S., and Wongmekiat, O. 2015. Enhancing phenolic contents and antioxidant potentials of Antidesma thwaitesianum by supercritical carbon dioxide extraction. Journal of Analytical Methods in Chemistry. 2015: 956298.
Puangpronpitag, D., Areejitranusorn, P., Boonsiri, P., Suttajit, M., and Yongvanit, P. 2008. Antioxidant activities of polyphenolic compounds isolated from Antidesma thwaitesianum Müll. Arg. seeds and Marcs. Journal of Food Science. 73(9): C648–C653.
Puangpronpitag, D., Yongvanit, P., Boonsiri, P., Suttajit, M., Areejitranusorn, P., Na, SK., et al. 2011. Molecular mechanism underlying anti-apoptotic and anti-inflammatory effects of Mamao (Antidesma thwaitesianum Müll. Arg.) polyphenolics in human breast epithelial cells. Food Chemistry. 127: 1450–1458.
Qin, T., Ren, Z., Liu, X., Luo, Y., Long, Y., Peng, S., et al. 2019. Study of the selenizing Codonopsis pilosula polysaccharides protects RAW264. 7 cells from hydrogen peroxide-induced injury. International Journal of Biological Macromolecules. 125: 534–543.
Re, R., Pellegrini, N., Proteggente, A., Pannala, A., Yang, M., and Rice-Evans, C. 1999. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biology and Medicine. 26(9–10): 1231–1237.
Rodrigues, E., Poerner, N., Rockenbach, I.I., Gonzaga, L.V., Mendes, C.R., and Fett, R. 2011. Phenolic compounds and antioxidant activity of blueberry cultivars grown in Brazil. Food Science and Technology. 31: 911–917.
Rupasinghe, H.V., Nair, S.V., and Robinson, R.A. 2014. Chemopreventive properties of fruit phenolic compounds and their possible mode of actions. Studies in Natural Products Chemistry. 42: 229–266.
Seeram, N.P. Berry fruits for cancer prevention: Current status and future prospects. 2008. Journal of Agricultural and Food Chemistry. 56(3): 630–635.
Thongchuai, B., Khamchun, S., Insuan, W., Daorueang, D., Sansai, P., and Insuan, O. 2022. Antidesma thwaitesianum Müll. Arg. fruit extract rich in 5-hydroxymethylfurfural exhibits anti-inflammatory effects in lipopolysaccharide-stimulated RAW264.7 macrophages. Journal of Herbmed Pharmacology. 11(2): 278–285.
Udomkasemsab, A., Ngamlerst, C., Adisakwattana, P., Aroonnual, A., Tungtrongchitr, R., and Prangthip, P. 2018. Maoberry (Antidesma bunius) ameliorates oxidative stress and inflammation in cardiac tissues of rats fed a high-fat diet. BMC Complementary and Alternative Medicine. 18: 1–11.
Udomkasemsab, A., Ngamlerst, C., Kwanbunjun, K., Krasae, T., Amnuaysookkasem, K., Chunthanom, P., et al. 2019. Maoberry (Antidesma bunius) improves glucose metabolism, triglyceride levels, and splenic lesions in high-fat diet-induced hypercholesterolemic rats. Journal of Medicinal Food. 22(1): 29–37.
Valko, M., Leibfritz, D., Moncol, J., Cronin, M.T., Mazur, M., and Telser, J. 2007. Free radicals and antioxidants in normal physiological functions and human disease. The International Journal of Biochemistry & Cell Biology. 39(1): 44–84.
Wang, W., Zhang, F., Li, Q., Chen, H., Zhang, W., Yu, P., et al. 2018. Structure characterization of one polysaccharide from Lepidium meyenii Walp., and its antioxidant activity and protective effect against H2O2-induced injury RAW264.7 cells. International Journal of Biological Macromolecules. 118: 816–833.
Xu, J.G., Hu, Q.P., and Liu, Y. 2012. Antioxidant and DNA-protective activities of chlorogenic acid isomers. Journal of Agricultural and Food Chemistry. 60(46): 11625–11630.
Yingngam, B., Monschein, M., and Brantner, A.H. 2016. Application of ultrasonic assisted extraction of bioactive compounds from the fruits of Antidesma puncticulatum Miq. and evaluation of its antityrosinase activity. Chiang Mai Journal of Science. 43(3): 519–533.
OPEN access freely available online
Natural and Life Sciences Communications
Chiang Mai University, Thailand. https://cmuj.cmu.ac.th
Supaporn Khamchun1,2, Natthapak Sillawatthumrong1, Wimonrut Insuan3, Wipob Suttana4, 5, Daoyot Daorueang1, Plubplung Sansai1 and Orapin Insuan1, *
1 Department of Medical Technology, School of Allied Health Sciences, University of Phayao, Phayao 56000, Thailand.
2 Unit of Excellence in Cellular and Molecular Immunodiagnosis and Therapy, School of Allied Health Sciences, University of Phayao, Phayao 56000, Thailand.
3 Department of Veterinary Technology, Faculty of Veterinary Technology, Kasetsart University, Bangkok 10900, Thailand.
4 Department of Biomedical Technology and Health Informatics, School of Health Science, Mae Fah Luang University, Chiang Rai, 57100, Thailand.
5 Biomedical Technology Research Group for Vulnerable Populations, Mae Fah Luang University, Chiang Rai, 57100, Thailand.
Corresponding author: Orapin Insuan E-mail: orapin.th@up.ac.th
Total Article Views
Editor: Nisit Kittipongpatana,
Chiang Mai University, Thailand
Article history:
Received: April 24, 2023;
Revised: August 17, 2023;
Accepted: August 21, 2023;
Online First: August 23, 2023

