
Nigella sativa Extract on Gingival Epithelium Exposed to LPS Porphyromonas gingivalis and Its Impact on the Expression of TLR-4 and NF-kB in vivo Study
Shafira Kurnia Supandi*, Andry Elvandari, Lambang Bargowo, and I Komang Evan WijaksanaPublished Date : August 21, 2023
DOI : https://doi.org/10.12982/NLSC.2023.061
Journal Issues : Number 4, October-December 2023
Abstract Porphyromonas gingivalis (P. gingivalis) is capable of enhancing oral dysbiosis resulting in an imbalance between beneficial commensal and periodontal pathogenic bacteria that causes chronic inflammation. Nigella sativa (N. sativa) is a type of herbal plant with antioxidant, anti-inflammatory, antimicrobial, anticancer, and other properties. One of the main active ingredients is thymoquinone. Researchers conducted this research to determine the impact of using Nigella sativa as a component in the prevention or treatment of periodontal disease. This research included a lab experiment using a randomized post-test-only control group design. The animals used were male white Wistar rats (Rattus norvegicus), aged 10-11 weeks, and weighing approximately 150-170 grams. All of them received adaptation for a week. Afterward, 45 of them were randomly allocated into three research groups: control group C (LPS P. gingivalis), group E1 (N. sativa extract + LPS), and group E2 (LPS + N. sativa). The rats were terminated after 7, 14, and 21 days and immunohistochemistry was performed using monoclonal antibody anti-TLR-4 and monoclonal antibody anti-NF-kB. The results showed that TLR-4 and NF-κB increased in group C and decreased in E1 and E2 groups. Although it was not statistically significant, the TLR-4 and NF-kB expression decreased more in the E2 group than in the E1 group. The result of this research shows that Nigella sativa can reduce the expression of TLR-4 and NF-kB as a part of the host’s innate defense.
Keywords: Nigella sativa, LPS Porphyromonas gingivalis, TLR-4, NF-kB, Periodontal disease
Citation: Supandi S.K., Elvandari, A., Bargowo, L., and Wijaksana, I.K.E. 2023. Nigella sativa extract on gingival epithelium exposed to LPS Porphyromonas gingivalis and its impact on the expression of TLR-4 and NF-kB in vivo study. Natural and Life Sciences Communications. 22(4): e2023061.
INTRODUCTION
The four main elements of the periodontium that support teeth to their function are the gingiva, periodontal ligament, cementum, and alveolar bone. The underlying tissue and the attachment apparatus are both protected by the gingiva. Gingiva epithelial cells respond to infection, signal subsequent host reactions, and combine innate and acquired immune responses as part of innate host defenses (Newman et al., 2019).
Gingivitis is the earliest stage of periodontal disease. Clinically apparent signs of gingival inflammation include redness, gingival enlargement, and bleeding on probing, although they do not result in attachment loss (Augustina et al., 2019). Gingivitis that is left untreated can destroy the tissues that support the teeth, including the gingiva, cementum, periodontal ligament, and alveolar bone (Senthilnathan et al., 2020). Periodontitis is periodontal disease that develops into a chronic, irreversible, inflammatory disease that destroys bone (Mekhemar et al., 2020).
The etiological element in the progression of periodontitis is subgingival dental plaque biofilm. One of the most well-known periodontal pathogens, Porphyromonas gingivalis (P. gingivalis), is an essential part of the subgingival dental plaque biofilm (Zheng et al., 2021). It is well recognized that P. gingivalis virulence factors have a wide range of effects on inflammation, both directly and indirectly destroying periodontal tissue. These virulence factors influenced oral microbiota coaggregation, biofilm formation, and dysbiosis. Virulence factors of P. gingivalis are fimbriae, hemolysin, hemagglutinins, capsule, outer membrane vesicles (OMVs), lipopolysaccharides (LPS), and gingipains (Aleksijević et al., 2022). P. gingivalis, Tannerella forsythia, and Aggregatibacter actinomycetemcomitans are some of the pathogens that cause periodontitis (Bui et al., 2019).
Recognizing that the disease has a wider range of effects and potential pathologies requiring awareness of its treatment and prevention is crucial. Dental cleanings performed by a dental professional are part of the treatment. It may also involve the use of antibiotics and periodontal surgery in some circumstances. Primary prevention is without a doubt the most crucial kind of care because it is not only the most efficient but also the most economical way to manage the disease and its complications (Mann et al., 2019).
Antimicrobial resistance is a phenomenon that has been linked to synthetic antimicrobial agents and antibiotics. However, because of their unfavorable side effects, which include tooth discoloration, altered taste, and high cost, modern chemotherapeutic agents do not always succeed in improving periodontal health. Synthetic agents can now be successfully replaced with natural substances (Eid Abdelmagyd et al., 2019).
Nigella sativa (N. sativa) is also known as black cumin, black seed, black caraway, habbatul sawda, habbatul barakah, kalanja. kalanji, and kalojeera, is a member of the Ranunculaceae family (Mekhemar et al., 2020). This plant is an effective natural substance for a variety of diseases, particularly for the management of eczema, asthma, inflammation, cough, and other flu-like symptoms. The seeds have diuretic, carminative, and deworming properties (Dalli et al., 2022). Thymoquinone, which possesses anti-inflammatory and antimicrobial effects, is the primary active ingredient in N. sativa. Thymoquinone prevents bacterial development, particularly gram-negative bacteria (Staphylococcus aureus, P. gingivalis). It suppresses inflammatory cytokines IL-1, IL-6, TNF-α and NF-kB, and TLR-4 (Tantivitayakul et al., 2020; Fitriatul et al., 2022).
Toll-like receptors (TLRs) are a subfamily of type I transmembrane proteins. TLR-4 plays a crucial role in inflammatory responses in periodontal tissues as it is the main pattern recognition receptor and signaling molecule in response to P. gingivalis virulence agents, such as lipopolysaccharide (Lu et al., 2018). NF-kB operates as a major modulator of pro-inflammatory gene activation. TLR-4/ NF-kB pathway is an essential signaling mechanism in immune response to microbial pathogens. It plays role in recognizing and responding to pathogen-associated molecular patterns (PAMPs) and activating immune response (Kuzmich et al., 2017). This research was conducted to be the basis for developing a strategy to prevent periodontal disease by suppressing inflammation using herbal ingredients.
MATERIALS AND METHODS
This experiment received ethical approval from the Ethical Committee of Dental Research Airlangga University (KKEPK) 2015 with the number 49/KKEPK.FKG/IV/2015.
Materials
Preparation of experimental animals
In total, 45 male Wistar rats (Rattus norvegicus) weighing 120-150 grams and aged 8-10 weeks, were used in this experimental in vivo research. The animals were divided into three groups: C (the control group after LPS P. gingivalis was injected), E1 (N. sativa was given before LPS P. gingivalis), and E2 (N. sativa was given after injection of LPS P. gingivalis). The animals underwent a clinical evaluation and spent 14 to 24 hours in a suitable environment. Particular tendencies and essential clinical indicators were examined to assess the health of the animals. Cage control and sustainability issues were handled by qualified and experienced staff from the Biochemistry Laboratory, Faculty of Medicine, Universitas Airlangga, and surrounding conditions, including food supplies, were acceptable.
Preparation of LPS from Porphyromonas gingivalis
Intracellular injections of Lipopolysaccharide 1435 / 1450 (tetra-acetylated) (Astarte Biologics, W A, USA, catalog number 7010) were used to induce immunoneuromodulation.
Preparation of Nigella sativa
The black cumin seeds are cleaned, dried, and blended. After being macerated in 90% ethanol for 96 hours, the products were combined, concentrated, and boiled in a water bath at 600 oC for 14 days to produce a pure extract, which was then kept in a petri dish covered in aluminum foil. Nigella sativa (N. sativa) was produced by diluting a 3% N. sativa extract.
Method
P. gingivalis LPS was given to the first group in this research as a control, while N. sativa extract was given to the second group four days before P. gingivalis LPS. The third group had both procedures done on the same day.
LPS Application
Gingivitis was induced in the animals intrasulcularly in this animal by injecting 1.0 μg/ml of the Pg LPS 1435 / 1450 on the buccal portion of the rats’ upper right molars.
Nigella sativa Extract Application Procedure
The gingiva surrounding the teeth received 0.5 ml of a 3% N. sativa extract twice a day for 21 days. In addition, routine assessments utilizing immunohistochemical peroxidase were performed on days 4, 7, and 21. C represents the control group after they were given LPS P. gingivalis, while in the E1 group, N. sativa extract was administered, and four days later, LPS P. gingivalis was injected into the rats' buccal upper right molars. In the E2 group, LPS P. gingivalis was administered first, and then at the same time N. sativa extract was injected.
Tissue Harvesting Procedure
Micro-tissue cutting was performed based on the guidelines for in vivo experiments at Faculty of Medicine’s Anatomical Pathology Laboratory Universitas Brawijaya.
Immunohistochemical
Immunohistochemical staining was used to examine TLR-4 and NF-κB expression from gingival tissues of a buccal portion of the rats’ upper right molars. TLR-4 and NF-κB expression was determined by measuring the number of cells expressing TLR-4 and NF-κB that was obtained from the gingival epithelium of Wistar rats. TLR-4 and NF-κB was assessed by an immunohistochemical technique using anti-rat TLR-4 and anti-rat NF-κB monoclonal antibody, by counting the number of cells that react positively to anti-rat TLR-4 and NF-κB monoclonal. The cells antibody was obtained from the gingival epithelium of Wistar rats. Cells were counted under a microscope with 400x magnification. Monoclonal antibodies against TLR4 (sc-30002) and NF-κB (p65 (F-6): sc-8008) of gingival tissue were obtained from Santa Cruz Biotechnology, Inc., USA. These procedures were done at the Microbiology Laboratory, Faculty of Dentistry, Universitas Airlangga.
Statistical Analysis
A statistical analysis of the group mean values and significant differences across the groups was performed using SPSS software with one-way ANOVA, and a P-value < 0.05 was considered statistically significant.
RESULTS
The outcomes of analyzing TLR-4 and NF-κB expression in gingiva exposed to P. gingivalis bacteria LPS before and after topically applying N. sativa extract on day 4, day 7 and day 21 of observation can be seen in Table 1.
Table 1. Mean and standard deviation of TLR-4 and NF-κB expression on gingiva given to LPS Pg before and after an N. sativa extract topical application on the day 4, day 7, and day 21 observation.
|
Variable |
Group |
Days |
||||||||
|
4 |
7 |
21 |
||||||||
|
Mean |
SD |
p |
Mean |
SD |
p |
Mean |
SD |
p |
||
|
TLR-4 |
C E1 E2 |
36.4 31.8 28.8 |
3.05 5.17 4.21 |
0.000* |
38.4 29.8 30.6 |
8.17 3.7 2.7 |
0.013* |
40.6 29.8 25.8 |
7.54 3.7 4.5 |
0.001* |
|
NFκB |
C E1 E2 |
29 24 23,2 |
2.92 1.87 1.92 |
0.000* |
28 20.8 19.4 |
4.18 0.84 1.14 |
0.000* |
28.8 20.8 16.4 |
2.39 0.84 1.14 |
0.000* |
Note: * = P < 0.05 is significantly different, C: Control group after given LPS Pg. E1 (Experiment 1): N. sativa extract was given before receiving LPS (Pg). E2 (Experiment 2): Both treatments were given at the same time.
The TLR-4 and NF-κB expression levels on days 4, 7, and 21 show a significant difference between groups C, E1, and E2 (Table 1). The means of groups E1 and E2 are decreased in comparison to group C.
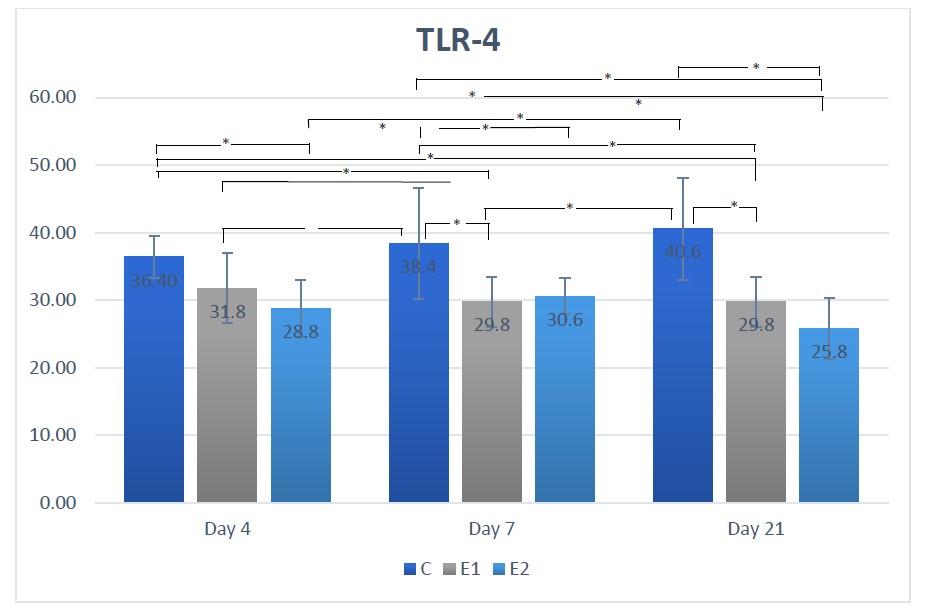
Figure 1. Means TLR-4 according to the day for each group.
Description: the sign * indicates there is a significant difference (P <0.05)
In contrast to the control group, the TLR-4 expression in the E1 and E2 groups was lower on days 4, 7, and 21. This is shown in Figure 1. As can be seen, there was no significant difference between the E1 and E2 groups on day 7, and the TLR-4 expression in the E2 group was significantly lower on days 4 and 21 compared to the E1 group.
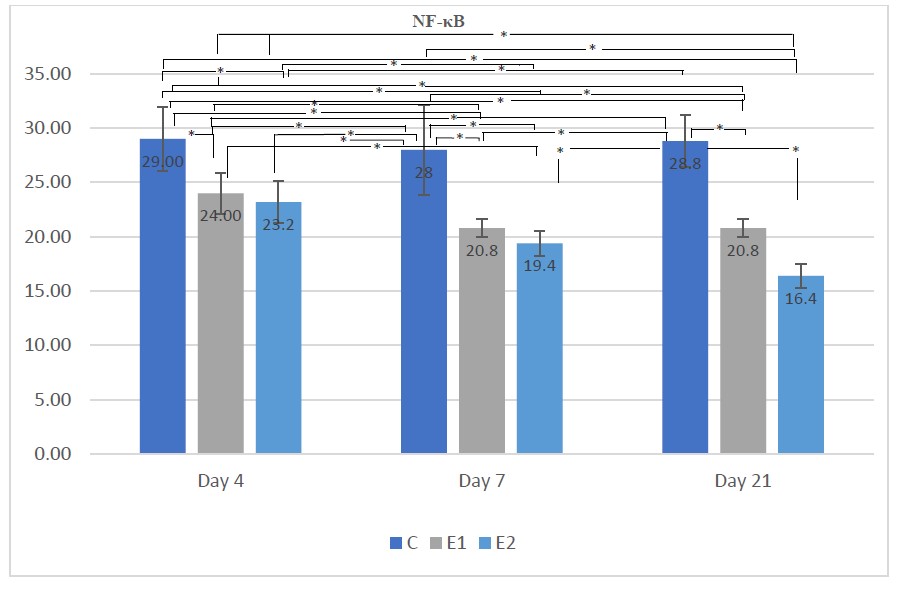
Figure 2. Means NF-κB according to the day for each group
Description: the sign * indicates there is a significant difference (P <0.05)
Figure 2 demonstrates that on days 4, 7, and 21 there was decreased NF-κB expression in the E1 and E2 groups compared to the control group. As can be observed, NF-κB expression in the E2 group on day 21 was significantly decreased compared to the E1 group, while there was no significant difference between the E1 and E2 groups on day 4 and day 7.
The rat gingival epithelium was subjected to a 400x magnification immunohistochemical analysis to assess the expression of TLR-4. The results are shown in Figure 3.

Figure 3. An overview of TLR-4 expression in rats’ gingival epithelial cells (black arrow).
(A) control, (B) N. sativa extract + LPS P.gingivalis, (C) LPS P.gingivalis + N. sativa extract performed at 400x magnification
The results of an NF-κB expression research of the rat gingival epithelium at 400x magnification are shown in Figure 4.
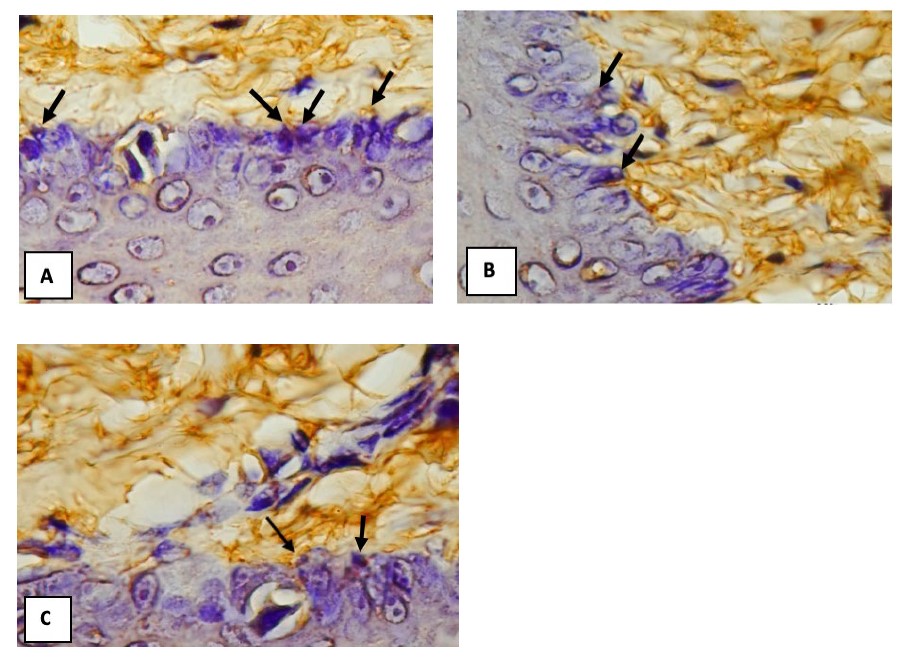
Figure 4. An overview of NF-κB expression in rats’ gingival epithelial cells (black arrow).
(A) control, (B) N. sativa extract + LPS P.gingivalis, (C) LPS P. gingivalis + N. sativa extract performed in 400x magnification.
DISCUSSION
The number of cells expressing TLR-4 was higher in the control group, which was stimulated with LPS P. gingivalis without a topical extract of N. sativa (Figure 1). This is associated with TLR-4 a pattern recognition molecule (PRM) that recognizes LPS, the primary glycolipids found on the surface of gram-negative bacteria. The toxicity of these bacteria is caused by lipid A (Ntoufa et al., 2016). Lipid A is present on the outer membrane of P. gingivalis, which aids in its interaction with the innate immune system (Zheng et al., 2021).
TLR-4 expression in the gingival epithelium was lower in the E1 and E2 treatment groups (Figure 1) compared to the control group, This is because the gingival epithelial cells act as a sensor that does not detect the presence of bacteria or bacterial products, such as LPS. The absence of this interaction between the PRR (Pattern Recognition Receptor) and PAMPs (Pathogen-associated Molecular Pattern) limits the activation of the intracellular calcium channel, which is linked to the absence of several molecular effectors. This is because TLR expression is regulated by cell type and type of stimulus (Ma’at, 2009). Thymoquinone is known to form irreversible compounds with nucleophilic amino acids in proteins, which can inactivate proteins and cause functional disruptions. Surface adhesins, cell wall polypeptides, and membrane-bound enzymes are its targets in bacterial cells (Cowan, 1999).
At each period, the mean TLR-4 expression in the E1 and E2 treatment groups was lower than in the C group as shown in Table 1 and Figure 1. This is by the theory that when the population of motile bacteria such as vibrio, spirilla, and spirochete increases on days 4 to 9, the ecology of plaque microorganisms becomes more complex. When plaque accumulation goes untreated for 10 to 21 days, the next stage of gingivitis symptoms appears (Samaranayake, 2018; Newman et al., 2019). On day 21, the control group generated solely by LPS P. gingivalis had an increased mean expression of TLR-4, whereas groups E1 and E2 had decreased mean expressions of TLR-4.
There are two pathways in the TLR-4 signaling system, one that is dependent on Myeloid differentiation factor 88 (MyD88) and one that is not. Inflammatory cytokines or TRIFs (Toll-IL-1R domain containing adapter-inducing interferons) are produced via pathways that rely on MyD88. TLR-3 and TLR-4 activate a MyD88-independent pathway that leads to IFN-β. MyD88 activation initiates a signaling cascade that results in continual kinase activation and translocation of key transcription factors such as nuclear factor (NF)-кB and interferon regulatory factor (IRF). Finally, MyD88 forms a complex with the TIR adapter-containing adaptor protein, which recruits IL-1 receptor-associated kinase and tumor necrosis factor (TRAF)-6, which activates the IкB kinase complex (IKK) (Yuana and Anum, 2011).
Table 1 and Figure 2 reveal that in each period, group C had the largest mean number of gingival epithelial cells expressing the most NF-кB. The activation of TLR ligands activates pathogen phagocytosis as well as inflammatory responses to phagosome content. The creation of a pro-inflammatory state mediated by specific cytokines and chemokines is the most essential aspect of TLR activation. The TIR domain-containing adapter-inducing interferon (IFN)-β (TRIF) adapter molecule is attracted to the intracellular moiety of TLR3 directly or to TLR4 via the TRIF-related adapter molecule (TRAM) after MyD88 tagging, resulting in the activation of the tank-binding kinase. TRAF-6 and TBK-1 both are places where an NF-кB-dominant immune response or an IRF-3-dominant immune response with a type 1 IFN activation pattern is induced (Liu and Zen, 2021).
The mean number of NF-кB expressing gingival epithelial cells was decreased in groups E1 and E2 compared to group C. According to N. sativa extract containing Thymoquinone provides anti- inflammatory properties by inhibiting NF-кB activity directly. Thymoquinone inhibits the phosphorylation of the inhibitory factor IкB kinase, which prevents NF-кB from entering the cell nucleus, reducing NF-кB activity and decreasing proinflammatory cytokine expression (Sethi et al. 2008). It is feasible that the N. sativa extract group (E1), a preventive, and the N. sativa extract group (E2), a curative, demonstrated a reduction in NF-кB expression (E2). This research needs further investigation to establish which of the extract Nigella sativa's active components has a major effect on the repair process.
CONCLUSION
This research showed TLR-4 and NF-kB expression on the Wistar rat’s gingival epithelium increased after stimulation with LPS P. gingivalis. This shows that TLR-4 and NF-кB play a key role in the gingiva's inflammatory response to pathogenic bacteria invasion. The expression of TLR-4 and NF-кB in the Wistar gingival epithelium was reduced after topical application of N. sativa extract. Therefore, N.sativa can be used as a preventive or curative treatment for periodontal disease.
ACKNOWLEDGMENTS
The authors thank the Faculty of Dental Medicine, Universitas Airlangga, Surabaya for their support.
AUTHOR CONTRIBUTIONS
Shafira Kurnia S assisted in conducting the experiments, performed the statistical analysis and data visualization, and wrote the manuscript. Andry Elvandari, Lambang Bargowo, I Komang Evan Wijaksana designed and conducted all of the experiments and wrote the manuscript. All authors have read and approved the final manuscript.
CONFLICT OF INTEREST
The authors declare that they hold no competing interests.
REFERENCES
Aleksijević, L.H., Aleksijević, M., Škrlec, I., Šram, M., Šram, M., and Talapko, J. 2022. Porphyromonas gingivalis virulence factors and clinical significance in periodontal disease and coronary artery diseases. Pathogens. 11(10): 1173. https://doi.org/10.3390/pathogens11101173
Asadi, H., and Ojcius, D.M. 2019. Association between periodontal pathogens and systemic disease. Biomedical Journal. 42(1): 27-35. https://doi.org/ 10.1016/j.bj.2018.12.001
Augustina, E.F., Sudiana, I.K., Soetjipto, and Rubianto, M. 2019. Expression of NF-κB and MMP-7 on defenses of the gingival epithelium injected lps porphyromonas gingivalis with the administration of curcumin. Journal of International Dental and Medical Research. 12(3): 941-946.
Bui, F.Q., Almeida-da-Silva, C.L.C., Huynh, B., Trinh, A., Liu, J., Woodward, J., Asadi, H., and Ojcius, D.M. 2019. Association between periodontal pathogens and systemic disease. Biomedical Journal. 42(1): 27-35. https://doi.org/10.1016/j.bj.2018.12.001
Cowan M.M. 1999. Plant products as antimicrobial agents. Clinical Microbiology Reviews. 12(4): 564-82. https://doi.org/10.1128/CMR.12.4.564.
Dalli, M., Bekkouch, O., Azizi, S.-e., Azghar, A., Gseyra, N., and Kim, B. 2022. Nigella sativa L. phytochemistry and pharmacological activities: A review (2019–2021). Biomolecules. 12 (1): 1-37. https://doi.org/10.3390/biom12010020
Eid Abdelmagyd H.A., Ram Shetty D.S., and Musa Musleh Al-Ahmari D.M. 2019. Herbal medicine as adjunct in periodontal therapies- A review of clinical trials in past decade. Journal of Oral Biology and Craniofacial Research. 9(3): 212-217. https://doi.org/10.1016/j.jobcr.2019.05.001
Fitriatul, N., Ismail, A., Ashraf Rostam, M., Fadhill, M., Jais, M., Affendi, M., Shafri, M., Faisal Ismail, A., and Hafiz Arzmi, M. 2022. Review: Exploring the potential of Nigella sativa for tooth mineralization and periodontitis treatment and its additive effect with doxycycline. IIUM Journal of Orofacial and Health Sciences, 3(1): 136–146. https://doi.org/10.31436/ijoh
Kuzmich, N.N., Sivak, K.V., Chubarev, V.N., Porozov Y.B., Savateeva-Lyubimova T.N., Peri F. 2017. TLR4 Signaling pathway modulators as potential therapeutics in inflammation and sepsis. Vaccines (Basel). 5(4): 34. https://doi.org/10.3390/vaccines5040034
Liu, M. and Zen, K. 2021. Toll-like receptors regulate the development and progression of renal diseases. Kidney Diseases. 7(1): 14–23. https://doi.org/10.1159/000511947
Lu, W., Gu, J. Y., Zhang, Y.Y., Gong, D.J., Zhu, Y.M., and Sun, Y. 2018. Tolerance induced by Porphyromonas gingivalis may occur independently of TLR2 and TLR4. PLOS ONE 13(7): e0200946. https://doi.org/10.1371/ journal.pone.0200946
Ma’at, S. 2009. Toll-like Receptor (TLR) and Natural Immunity. Indonesian Journal of Clinical Pathology and Medical Laboratory. 15(3): 111–116. https://doi.org/10.24293/ijcpml.v15i3.978.
Mann, J., Bernstein, Y., and Findler, M. 2020. Periodontal disease and its prevention, by traditional and new avenues. Experimental and Therapeutic Medicine 19(2): 1504-1506. https://doi.org/10.3892/etm.2019.8381
Mekhemar, M., Hassan, Y., and Dörfer, C. 2020. Nigella sativa and Thymoquinone: A natural blessing for periodontal therapy. Antioxidants (Basel). 9(12): 1260. https://doi.org/10.3390/antiox9121260
Newman, M.G., Takei, H.H., and Klokkevold, P.R. 2019. Newman and Carranza’s Clinical Periodontology 13th ed (F. A. Carranza & Dr Odont, Red.). Elsevier.
Ntoufa, S., Vilia, M.G., Stamatopoulos, K., Ghia, P., and Muzio, M. 2016. Toll-like receptors signaling: A complex network for NF-κB activation in B-cell lymphoid malignancies. Seminars in Cancer Biology. 39: 15-25. https://doi.org/ 10.1016/j.semcancer.2016.07.001
Samaranayake, L. 2018. Essential microbiology for dentistry (5th ed). Elsevier.
Senthilnathan, K., Ilango, P., Abirami, T., Vummidi, V., Mahalingam, A., and Reddy, V. 2020. Evaluation of antibacterial activity of Nigella sativa seed extract against Porphyromonas gingivalis and Prevotella intermedia. Journal of Interdisciplinary Dentistry. 10(2): 51. https://doi.org/10.4103/ jid.jid_62_19
Sethi, G., Kwang, S. A., and Aggarwal, B. B. 2008. Targeting nuclear factor-κB activation pathway by Thymoquinone: Role in suppression of antiapoptotic gene products and enhancement of apoptosis. Molecular Cancer Research. 6(6): 1059–1070. https://doi.org/10.1158/1541-7786.MCR-07-2088
Tantivitayakul, P., Kaypetch, R., and Muadchiengka, T. 2020. Thymoquinone inhibits biofilm formation and virulence properties of periodontal bacteria. Archives of Oral Biology, 115. https://doi.org/10.1016/j.archoralbio.2020.104744
Yuana and Anum, Q. 2011. Peranan toll-like receptor terhadap infeksi bakteri pada kulit. MDVI. 39(1): 42–48.
Zheng, S., Yu, S., Fan, X., Zhang, Y., Sun, Y., Lin, L., Wang, H., Pan, Y., and Li, C. 2021. Porphyromonas gingivalis survival skills: Immune evasion. Journal of Periodontal Research 56(6): 1007-1018. https://doi.org/10.1111/jre.12915
OPEN access freely available online
Natural and Life Sciences Communications
Chiang Mai University, Thailand. https://cmuj.cmu.ac.th
Shafira Kurnia Supandi*, Andry Elvandari, Lambang Bargowo, and I Komang Evan Wijaksana
Department of Periodontology, Faculty of Dental Medicine, Universitas Airlangga, Surabaya, Indonesia.
Corresponding author: Shafira Kurnia Supandi E-mail: shafira-k-s@fkg.unair.ac.id
Total Article Views
Editor: Anak Iamaloon,
Chiang Mai University, Thailand
Article history:
Received: September 19, 2022;
Revised: July 20, 2023;
Accepted: July 24, 2023;
Online First: August 21, 2023

