
Formulation Development and Evaluation of Chewable Gels Containing Jatuphalathika Extract
Aurasorn Saraphanchotiwitthaya*, Natthapat Saibuathong, Thanaphon Boonsirirungrueng and Pattana SripalakitPublished Date : August 18, 2023
DOI : https://doi.org/10.12982/NLSC.2023.060
Journal Issues : Number 4, October-December 2023
Abstract Jatuphalathika (JP) is composed of the fruits of four herbal plants, Terminalia chebula Retz., Terminalia bellirica Roxb., Terminalia arjuna Wight and Arn., and Phyllanthus emblica L. JP formula is traditionally used for fever, as a carminative, for detoxification, and as an anti-hyperlipidaemic. However, consumption of JP is restricted due to its unpleasant flavours, which include sour, bitter, and astringent notes. The purpose of the current study was to formulate chewable gels with JP extract and evaluate their stability. Different types and concentrations of gelling agents and flavouring agents were used in the formulation. The finished products without or with a package (foil-wrapped or vacuum-sealed) were stored at room temperature (30 ± 2°C), in a refrigerator (2–8 °C) for 1 month, and under thermal cycling between 2–8 °C for 48 h and 45 °C for 48 h for 4 cycles. Stability testing of the chewable gels was carried out. Among six formulations, JP chewable gel containing gelatin, stevioside syrup, sorbitol solution, and paraben concentrate without (F5) or with menthol (F6) gave the best results. Both formulations maintained the same texture when stored in vacuum-sealed packaging: F5 at room temperature or in the refrigerator, and F6 at room temperature. Under these storage conditions, they were physically, chemically, and microbiologically stable with acceptable taste and scent. It may be concluded that chewable gels F5 and F6 are easy-to-use formulations which could improve patient or customer compliance when using JP.
Keywords: Gallic acid, Gelatin, Obesity, Phytoconstituents, Stability, Stevia
Funding: This work was supported by Naresuan University (NU), and National Science, Research and Innovation Fund (NSRF) (Grant No. R2565B021).
Citation: Saraphanchotiwitthaya, A., Saibuathong, N., Boonsirirungrueng, T. and Sripalakit, P. 2023. Formulation development and evaluation of chewable gels containing jatuphalathika extract. Natural and Life Sciences Communications. 22(4): e2023060.
INTRODUCTION
Presently, natural-based ingredients from herbs in food supplements, cosmetics, and pharmaceutics have increased in popularity due to their biological activity, low toxicity, and environmental friendliness (Polshettiwar et al., 2022). However, the consumption of herbs is still limited since the available formulations are not acceptable for certain consumers, especially young children, the elderly, and patients with dysphagia (Liu et al., 2014). Some herbal products in tea form are inconvenient to use while herbal capsules and tablets may cause trouble when swallowing. Crushing tablets or mixing them with water or food before being taken may increase the risk of dose error, leading to health problems, medical treatment costs, or even death. Moreover, crushing tablets may make the unpleasant taste of herbs such as bitter, sour, or astringent notes more prominent (Prakash et al., 2014).
Although there are optional formulations which are more convenient for swallowing, such as elixirs, suspensions, and oro-dispersible tablets, elixirs require complicated solubilization techniques, while the solubilization of the active ingredient may make its apparent unpleasant taste even more noticeable. The texture and viscosity of suspensions may not be very satisfying to the patient. Likewise, the dissolving of oro-dispersible tablets may result in oral discomfort from a powdery sensation or dry mouth (Dille et al., 2018). As a result, a chewable gel formulation is chosen as a substitute formulation to address these issues.
The chewable gel or ‘gummy’ is an oral formulation comprising gelling agents, sweeteners, and flavouring agents. Preservatives and stabilizers may be added as necessary. A good chewable gel formulation should be firm, flexible, easily chewable, and swallowable. Moreover, it must be effective in masking the undesirable taste of the drugs or active ingredients (Davydova, 2018). This formulation is gaining attention due to lacking a complicated solubilization process, stability challenges and dosing errors. Moreover, its pleasant taste and ease of administration with no need to drink water could improve patient compliance (Rodríguez-Pombo et al., 2022). Moreover, many people prefer gummy products to pills due to their fruity flavors and candy-like taste. This is one of the reasons why they appeal to children who may otherwise be picky eaters and obese people who prefer the consumption of snacks, sweets, and soft drinks. Evidence currently suggests that chewing may decrease self-reported hunger and food intake, possibly through alterations in gut hormone responses related to satiety (Miquel-Kergoat et al., 2015).
Several chewable gel preparations have been investigated. Oral soft chewable jelly containing flurbiprofen was prepared. FP2 consisting of 4.5% pectin and 40% w/v sucrose was the optimum formula that presented a high percentage of dissolution efficiency (78.95%) and better consistency during handling. This formulation could be considered a promising dosage form for the improvement of patient compliance and drug solubility (Sabri et al., 2022). Moreover, the taste masking of ibuprofen soft-chew tablets was achieved by controlling pH at 4.5 and keeping the processing temperature below the crystalline-to-amorphous transition temperature. The formulation demonstrated good stability for up to 24 months and exhibited comparable dissolution to oral tablets. The findings indicate that several active pharmaceutical compounds are capable of producing chewable gels that are simple to swallow, well-tasted, and have a long shelf life (Morten et al., 2017).
Jatuphalathika (JP) is composed of the fruits of four herbal plants, Terminalia chebula Retz., Terminalia bellirica Roxb., Terminalia arjuna Wight and Arn., and Phyllanthus emblica L. It has been used in conventional medicines for fever, eye diseases, and health promotion. JP extract has been shown to reduce hyperlipidaemia, total cholesterol, and triglyceride levels in hypercholesterolaemic rats in previous studies (Rinthong et al., 2016). The capability of each herbal component in the JP formula to reduce blood cholesterol levels has also been confirmed (Thakur et al., 1988; Gupta et al., 2001; Patil et al., 2011; Pathompak et al., 2015). These results demonstrated the JP formula’s effectiveness in treating hyperlipidaemia and possible use to control weight. Unfortunately, the taste of the JP extract is sour, astringent, and bitter. Therefore, the development of JP in chewable gel forms which can be adjusted the taste profile according to customer preferences and hide the sensory properties of undesirable substances could overcome this limitation.
As a result, producing a JP chewable gel is incredibly challenging in order to produce a finished product that is stable, palatable, and easy to consume. The present investigation aimed to develop chewable gel tablets with JP extract and to study the physical, chemical and biological stability of the finished products in various packaging under the determined storage conditions.
MATERIALS AND METHODS
Materials
Dried fruit of T. chebula Retz., T. bellirica Roxb., T. arjuna Wight and Arn., and P. emblica L. was purchased from a local herbal store in Phitsanulok, Thailand. Microscopic authentication of all dried fruits and their powders was performed by the Department of Medical Sciences Ministry of Public Health, Nonthaburi, Thailand. Gelatin, gellan gum, pectin, and sorbitol solution were purchased from Chemipan, Thailand. The stevioside solution was obtained from Green Foods Asia Co., Ltd. Gallic acid was purchased from Sigma-Aldrich (St. Louis, MO, USA).
Preparation of JP extract
Dried fruit of T. chebula Retz., T. bellirica Roxb., T. arjuna Wight and Arn., and P. emblica L. was ground into powder using a blender. Equal portions of the four herbal powders of 80 g each were mixed and extracted by decoction (60–70°C) in 1,500 ml of distilled water for 2 h with occasional stirring. After that, the herbal mixture was filtered through filter paper, then the filtrate was concentrated using a rotatory evaporator and hot air oven (60–70°C) to complete drying for 48 h. The percentage yield of the JP extract (%w/w of dried herb) was calculated. The extract was kept in a refrigerator (2–8°C) for further analysis and testing.
Determination of gallic acid content by HPLC analysis
The gallic acid (GA) content of the JP extract was assessed using the HPLC method (Kardani et al., 2013). The HPLC system (Shimadzu, Kyoto, Japan) included an LC-20AT pump, an SPD-20A UV detector equipped with an SPD-20A system controller, and an SIL-10ADVP sample injector with a 20 μl sample loop. A C18 column (250 mm × 4.6 mm i.d., 5 μm, 250°A) (ACE®, Scotland) was used for the chromatographic separations. The mobile phase was a 90 : 10 v/v mixture of water and acetonitrile containing 1.0 %v/v orthophosphoric acid, pH 3.00. The separations were carried out isocratically at a flow rate of 1 ml/min. The column was maintained at room temperature (27 ± 2 °C). A UV detector at a wavelength of 210 nm was employed to identify the peaks. GA standard solutions at concentrations ranging from 1 to 100 μg/ml in the mobile phase were prepared. JP extract was diluted in the mobile phase to produce a 1 mg/ml test solution. Before being injected into the HPLC system, the standard solution or test samples were filtered via a 0.45 mm membrane filter (Millipore, MA, USA). The components were identified by comparing the retention times of the observed peaks to those of the original reference GA standard. The calibration curve of pure standards was used to determine the amount of GA.
For the determination of GA in JP chewable gels, one tablet of JP chewable gel was heated (at 60 °C) in a water bath until it had completely melted, and then distilled water was added to a final volume of 10 ml. The mixture was diluted to 1 mg/ml in distilled water. The test solution was filtered through a 0.45 μm membrane filter and analysed for GA content using the same HPLC technique as for analysis of GA in the extract.
Determination of gelling agents in a chewable gel base
The specifications of a chewable gel require good physical appearance, a non-sticky texture with good gel-forming ability, no syneresis, the capability to mask the bitter and sour taste of JP extract, and stability under determined storage conditions. To determine an appropriate gelling agent in the formulation, the chewable gel base was formulated using different types and concentrations of gelling agents in distilled water, mixed with 10% w/w sorbitol solution and 1 %w/w paraben concentrate (20 %w/w methylparaben and 1 %w/w propylparaben in propylene glycol). Gelatin (10, 12, and 15 %w/w), gellan gum (1.5, 1.75, and 2 %w/w), or pectin (1.2, 1.4, and 1.6 %w/w) were used as gelling agents (Prakash et al., 2014). The chewable gel base which produced the best texture, measured by visual observation and organoleptic methods, without syneresis was selected and further applied in JP chewable gel.
Formulation of JP chewable gels
JP chewable gels consisted of a gelatin base with the addition of 4.32 %w/w JP extract, flavouring agents (menthol, citric acid) and sweetening agents (1 %w/w steviol glycoside in maltitol syrup). Menthol was dissolved in a small amount of 95 %v/v ethanol while citric acid was dissolved in distilled water before mixing. JP chewable gels were prepared by dispersing gelling agents in distilled water, stirring to mix well, and storing for 10 min for swelling. The dispersion was heated in a water bath to 60 °C and mixed homogeneously with sorbitol solution, stevioside solution, citric acid, and paraben concentrate, respectively. When the temperature cooled down to 50 °C, menthol was added and stirred to mix well. The formulation was poured into a silicone mould and allowed to cool down to room temperature (Čižauskaite et al., 2019). Finally, the settling cubic JP chewable gels were obtained and further tested for their stability.
Evaluation of JP chewable gels
Texture analysis
The texture of the freshly prepared JP chewable gel tablets was analysed compared with the formulations stored at room temperature (27 ± 2 °C) or in a refrigerator (2–8 °C) for 1 month. The hardness, springiness, cohesiveness, gumminess, and chewiness of the chewable gels were measured using a texture analyser (TA-XT plus, Stable Micro Systems, USA). The parameters of the test were: cylinder probes 20 mm, strain 50%, test speed 1.00 mm/s, hold time 3 s, and trigger force 2.0 g (Suwan et al., 2017).
Organoleptic evaluation
The organoleptic properties of the chewable gel were preliminarily evaluated. The physical appearance of chewable gels including colour, shape, and clarity was evaluated by visual observation. The texture was evaluated by rubbing and compressing the chewable gels between two fingers.
Syneresis test
Syneresis is the spontaneous contracting or shrinking of a gel, accompanied by the expulsion of liquid. The chewable gel base was kept at room temperature (25 ± 2 °C) for 24 h, then the size was measured using a Vernier calliper. Any formulation which had a significant difference between the initial and final size indicated syneresis and was rejected.
pH measurement
A chewable gel sample (0.5 g) was heated (at 60 °C) in a water bath until it had completely melted. Distilled water was added to make a final volume of 50 ml. pH measurement of the solution at room temperature (27 ± 2 °C) was performed using a pH meter.
Stability testing
JP chewable gels were packed in three different forms: foil-wrapped tablets, tablets in a vacuum-sealed package, and unwrapped tablets in a tightly closed glass bottle. They were stored under determined storage conditions as follows: room temperature (27 ± 2 °C) for 1 month, refrigerator (2–8 °C) for 1 month, 40 °C for 1 month and thermal cycling of 2–8 °C for 48 h then 45 °C for 48 h for 4 cycles (Prakash et al., 2014). At the end of the storage times, JP chewable gels under various storage packaging and conditions were evaluated compared with the freshly prepared product.
Microbial contamination testing
Microbial contamination of JP chewable gels was evaluated by visual observation and the pour plate technique. For the pour plate technique, sterile nutrient agar was prepared using an autoclave (121 °C, 15 min), then the agar was allowed to cool to 45 °C. The chewable gel was rinsed with 1 ml of sterile distilled water and poured into a Petri dish, follow by sterile nutrient agar. The plate was gently rotated in a circular motion to achieve uniform distribution and incubated at 37 °C for 48 h. The microbial growth of JP chewable gel was observed compared to the control.
Statistical analyses
Statistical analyses were conducted by one-way ANOVA. Post hoc comparisons of the means were performed according to Turkey’s HSD test; P < 0.05 was regarded as statistically significant (n = 3).
RESULTS
Preparation of herbal test samples
The mixture of dried powder from the fruits of T. chebula Retz., T. bellirica Roxb., T. arjuna Wight and Arn., and P. emblica L. was extracted by decoction in distilled water. The percentage yield of the JP extract was 26.8 %w/w of dried powder. The physical appearance was a deep brown viscous extract with a unique herbal scent (Figure 1).
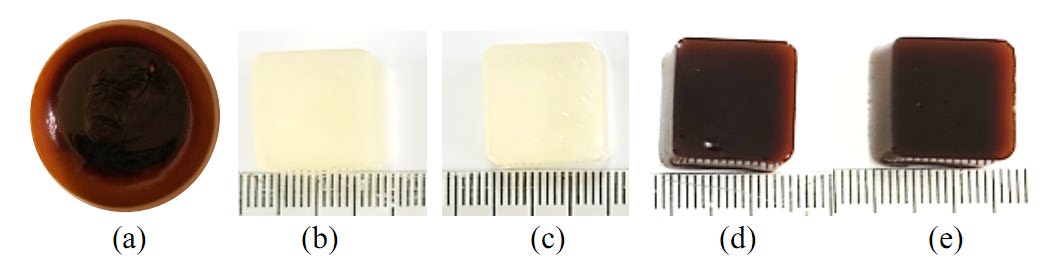
Figure 1. The physical appearance of (a) JP extract, chewable gel base of (b) F5, (c) F6, and JP chewable gel (d) F5 and (e) F6.
Determination of GA by HPLC analysis
The HPLC analysis indicated that GA is one of the major polyphenolic compounds of JP extracts. The retention time of GA was approximately 5.0 min (Figure 2). From the results of the GA calibration curve a regression equation was obtained: y = 140645x − 20285, with r2 = 0.9999. The GA content in the extracts was calculated based on comparing retention times of the peaks according to the GA standard. The percentage yield of GA in dried herbs was 0.62 %w/w.


Figure 2. HPLC chromatogram of (a) gallic acid (100 µg/ml), (b) JP extract (1 mg/ml), the freshly prepared formulations of (c) F5 chewable gel base, (d) F5, (e) F6 chewable gel base, (f) F6 compared with (g) F5 chewable gel base, (h) F5, (i) F6 chewable gel base, (j) F6 kept at room temperature for 1 month in vacuum-sealed packaging.
Determination of gelling agents in a chewable gel base
Appropriate types and concentrations of gelling agents in JP chewable gel bases were studied. Gelatin, gellan gum, and pectin at various concentrations were used in the formulation to form gels. The physical appearance of chewable gel bases formulated from all three gelling agents was a clear gel. Gelatin at all concentrations (8, 10, and 12 %w/w) produced a base with good gel-forming ability, while bases containing gellan gum and pectin were unable to maintain their original shape over time. By organoleptic evaluation, it was found that using gelatin at higher concentrations resulted in higher firmness and lower softness (Table 1). Similarly, the hardness, gumminess, springiness, and chewiness of chewable gelatin bases tended to increase, while the cohesiveness was unchanged when assayed by the texture analyser at higher concentrations of gelatin (Table 2). For syneresis testing, a chewable gelatin base using 8, 10, and 12 %w/w gelatin was formulated and stored at room temperature (27 ± 2 °C) or refrigerator temperature (2–8 °C) for 24 h. At the end of the storage time, the size of chewable gels was measured. Only the chewable gelatin base containing 8 %w/w gelatin when kept at room temperature exhibited syneresis and was rejected from the study. For the formulation of JP chewable gel, 12 %w/w gelatin which provided more structural gel forming was used in the study.
Table 1. Organoleptic evaluation of chewable gel base.
|
Gelling agent (%w/w) |
Clarity |
Springiness |
Softness |
Firmness |
|
|
Gelatin |
8.00 |
+++ |
+ |
+++ |
+ |
|
10.00 |
+++ |
+++ |
++ |
++ |
|
|
12.00 |
+++ |
++ |
++ |
+++ |
|
|
Gellan gum |
1.50 |
+++ |
n.d. |
n.d. |
n.d. |
|
1.75 |
+++ |
n.d. |
n.d. |
n.d. |
|
|
2.00 |
+++ |
n.d. |
n.d. |
n.d. |
|
|
Pectin |
1.20 |
+++ |
n.d. |
n.d. |
n.d. |
|
1.40 |
+++ |
n.d. |
n.d. |
n.d. |
|
|
1.60 |
+++ |
n.d. |
n.d. |
n.d. |
|
Note: n.d. = not determined, (+) = low, (++) = moderate, (+++) = high.
Table 2. Texture evaluation of chewable gelatin base by texture analyser.
|
Gelatin (%w/w) |
Hardness (g) |
Springiness |
Cohesiveness |
Gumminess (g) |
Chewiness (g) |
|
8 |
514.57 ± 31.17 |
0.88 ± 0.09 |
0.91 ± 0.03 |
469.60 ± 17.48 |
411.93 ± 57.18 |
|
10 |
1077.90 ± 34.30 |
0.96 ± 0.01 |
0.91 ± 0.02 |
984.32 ± 56.23 |
948.77 ± 64.24 |
|
12 |
1436.02 ± 51.14 |
0.93 ± 0.04 |
0.91 ± 0.03 |
1305.01 ± 82.99 |
1212.40 ± 102.57 |
Formulation of JP chewable gel
Formulations F1–F4 were prepared as presented in Table 3. Their physical appearances were square-shaped, size 1.4×1.4×0.8 cm and brown in colour, with good firmness and clarity. However, mixing of JP extract and other ingredients into a chewable gel base resulted in the formulation being too soft, and the firmness was noticeably reduced over time, especially when citric acid was included. Therefore, two other formulations were developed by increasing the gelatin content to 15 %w/w, without the addition of citric acid, without (F5) or with menthol (F6), respectively (Table 3). The results showed that the freshly prepared F5 and F6 had no syneresis. Both formulations were further evaluated for stability testing. Their organoleptic evaluation is shown inTable 4. The physical appearance of the chewable gel base and JP chewable gel formulations F5 and F6 were presented in Figure 1.
Table 3. Ingredients of JP chewable gel formulations.
|
Ingredient (g) |
F1 |
F2 |
F3 |
F4 |
F5 |
F6 |
|
JP extract |
2.16 |
2.16 |
2.16 |
2.16 |
2.16 |
2.16 |
|
Gelatin |
6.0 |
6.0 |
6.0 |
6.0 |
7.5 |
7.5 |
|
Stevioside solution |
21.0 |
21.0 |
21.0 |
21.0 |
21.0 |
21.0 |
|
Sorbitol solution |
5.0 |
5.0 |
5.0 |
5.0 |
5.0 |
5.0 |
|
Citric acid monohydrate |
- |
- |
0.375 |
0.375 |
- |
- |
|
Menthol |
- |
0.15 |
- |
0.15 |
- |
0.15 |
|
95 %w/w ethanol |
- |
0.15 |
- |
0.15 |
- |
0.15 |
|
Paraben concentrate |
0.5 |
0.5 |
0.5 |
0.5 |
0.5 |
0.5 |
|
Distilled water to |
50.0 |
50.0 |
50.0 |
50.0 |
50.0 |
50.0 |
Table 4. Organoleptic evaluation of freshly prepared JP chewable gel formulations.
|
Formula |
Appearance |
Springiness |
Softness |
Firmness |
|
F1 |
Clear brown cube |
++ |
++ |
+++ |
|
F2 |
Clear brown cube |
++ |
++ |
+++ |
|
F3 |
Clear brown cube |
+ |
+++ |
+ |
|
F4 |
Clear brown cube |
+ |
+++ |
+ |
|
F5 |
Clear brown cube |
++ |
++ |
+++ |
|
F6 |
Clear brown cube |
++ |
++ |
+++ |
Note: (+) = low, (++) = moderate, (+++) = high.
Stability testing of JP chewable gels
The freshly prepared F5 and F6 chewable gels were wrapped in foil, vacuum-sealed, or kept as unwrapped tablets in a tightly closed glass bottle. Both formulations were stored at room temperature (27 ± 2 °C), at 2–8 °C for 1 month, or under thermal cycling. Their stability was assessed compared with the freshly prepared formulation. Both formulations when kept at 40 °C for 1 month and under thermal cycling exhibited slight deformation and were not further evaluated. Testing of their chewable gel base without JP extract produced similar results but retained a slightly more shape.
After storage at room temperature (27 ± 2 °C) and 2–8 °C for 1 month, formulations F5 and F6 were evaluated for physical, chemical, and biological stability. The physical appearance of both formulations and their chewable gel base was unchanged; the square-shaped gel with a brown colour for JP chewable gels, and the clear gel for chewable gels base showed good firmness with no syneresis. However, packaging in foil caused some creases on the gel surface, while gels kept in a vacuum sachet were slightly flattened.
Their texture which was evaluated by organoleptic testing was unchanged, except for the foil-wrapped formulation which had a rigid texture which was stickier and harder. This was similar to the results from chewable gel base in bare tablets. By texture analysis, the hardness, gumminess, and chewiness of chewable gels F5 and F6 in foil-wrapped packages or bare tablets kept at room temperature (27 ± 2 °C) and 2–8 °C altered significantly over time. Both formulations maintained the same texture when stored in a vacuum-sealed package, F5 at room temperature or in the refrigerator, and F6 at room temperature. Texture evaluation of JP chewable gels (F5 and F6) after storage is presented in Figure 3.

Figure 3. Texture evaluation of JP chewable gels (F5 and F6) kept under determined storage conditions (RT: room temperature 27 ± 2 °C, CT: cool temperature 2–8 °C) for 1 month; (a) hardness, (b) springiness, (c) cohesiveness, (d) gumminess, and (e) chewiness.
The pH value of formulations F5 and F6 after storage was approximately 6.0–6.2, while the pH value of their chewable gel base was 6.5-6.8. This is close to the pH value of freshly prepared products. Determination of GA content by HPLC technique revealed that both formulations F5 and F6 were chemically stable due to there being no significant difference compared to the freshly prepared product (Figure 2). By visual observation, there was no microbial growth in F5 and F6 chewable gels and their chewable gel base which were kept under the determined storage conditions. This is in accordance with the results obtained using the pour plate technique. Table 5 presents a stability assessment of JP chewable gels (F5 and F6) that were stored under specific storage conditions for a month.
Table 5. Stability evaluation of JP chewable gels (F5 and F6) stored for 1 month.
|
Formula |
Storage condition |
Appearance |
pH |
Gallic acid content (mg/tablet) |
Microbial growth |
|
|
F5 |
Freshly prepared |
Clear brown cube |
6.1 |
2.25 ± 0.02 |
No |
|
|
Foil-wrapped |
27 ± 2 °C |
Clear brown cube |
6.2 |
2.11 ± 0.02 |
No |
|
|
2–8 °C |
6.1 |
1.93 ± 0.01 |
No |
|||
|
Vacuum-sealed |
27 ± 2 °C |
6.0 |
2.24 ± 0.01 |
No |
||
|
2–8 °C |
6.2 |
2.26 ± 0.04 |
No |
|||
|
No package |
27 ± 2 °C |
6.0 |
2.15 ± 0.05 |
No |
||
|
2–8 °C |
6.2 |
2.14 ± 0.01 |
No |
|||
|
F6 |
Freshly prepared |
Clear brown cube |
6.0 |
2.18 ± 0.04 |
No |
|
|
Foil-wrapped |
27 ± 2 °C |
Clear brown cube |
6.2 |
2.13 ± 0.03 |
No |
|
|
2–8 °C |
6.1 |
2.14 ± 0.04 |
No |
|||
|
Vacuum-sealed |
27 ± 2 °C |
6.0 |
2.21 ± 0.01 |
No |
||
|
2–8 °C |
6.2 |
2.16 ± 0.01 |
No |
|||
|
No package |
27 ± 2 °C |
6.1 |
2.17 ± 0.01 |
No |
||
|
2–8 °C |
6.0 |
2.15 ± 0.00 |
No |
|||
DISCUSSION
In the current investigation, JP extract was produced by decoction and gallic acid contained in the extract was detected by HPLC analysis. In general, each JP capsule was filled with approximately 450 mg of JP powder. Based on this information, JP chewable gel containing 0.12 g of JP extract per tablet was prepared to obtain the equivalent dose.
Appropriate types and concentrations of gelling agents in JP chewable gel bases were studied. Among three gelling agents, gelatin at high concentration produced a base with good gel-forming ability. By texture analysis, the hardness, gumminess, springiness, and chewiness of chewable gelatin bases tended to increase with the dose-response relationship, while the cohesiveness was unchanged. In addition, JP chewable gel high concentration of gelatin provided more structural gel forming with no syneresis. The results are in accordance with those of a previous study, which reported that the texture profile of gelatin-based chewable tablets improves with an increasing gelatin concentration (Banjongsinsiri et al., 2020). Nevertheless, our results are different from those of the studies by Čižauskaite et al. (2019) and Prakash et al. (2014) which reported that gellan gum and pectin at the same concentrations produce a good gel formation. These may be due to the differences in ingredients, pH, and sources of gelling agents.
Gelatin is a protein-based gelling agent extracted from animals. Since gelatin easily forms a stable gel texture, it is widely applied in chewable gel production (Dille et al., 2018). For further study, 12 %w/w gelatin was used in the formulation incorporating JP extract.
Since JP extract provides a sour, astringency and slightly bitter taste. Therefore, sweetening agents and flavoring agents were used in the formulation. Sugar-free food substituted with artificial sweeteners is now very popular due to its low-calorie value. The U.S. Food and Drug Administration has approved several artificial sweeteners such as saccharin, acesulfame-K, sucralose, aspartame, etc. as per acceptable daily intake value (ADI) (Chattopadhyay et al., 2014). In the previous investigation, xylitol which is widely used in the food industry has been applied as an artificial sweetener in the development of gummy jelly incorporated with Lysiphyllum strychnifolium leaf extract (Thilavech et al., 2023). In the current study, stevioside and maltitol, which have been considered to be healthy sugar substitutes, were used as non-caloric sweetening agents, in addition to sorbitol solution. Citric acid or menthol was used as flavouring agents.
In the formulation of JP chewable gels, F1–F4 containing 12%w/w gelatin and various additives were prepared. F1 with a sour and astringency taste from the extract was blended with a sweetening agent. Menthol was added in F2, expecting for cooling and refreshing effect when chewing, and masking effect on astringency due to a slight numbness effect on the taste buds. F3 was added with citric acid, expecting a sourer taste which blended well with the sweetness and masked the herbal scent. For F4, both menthol and citric acid were added to the formulation. The obtained chewable gel was predicted to have a sweet and prominent sour taste with a cooling and refreshing sensation. However, the obtained JP chewable gels being too soft and the reduction of firmness over time was observed, especially when citric acid was included. This may be due to the effect of citric acid and some phenolic acid in JP extract. The previous investigation has reported that the addition of lactic acid (LA), citric acid (CA) or malic acid (MA) in gelatin dispersions greatly affected the gel strength and phase transition temperatures. Higher concentrations of LA, CA, or MA exhibited the weakening effects of these acids on junction zones of the gelatin network in aqueous media. In addition, different acid types affected various rheological properties of gelatin dispersions (Zhou et al., 2022).
To develop JP chewable gel with good firmness, citric acid was withdrawn, and the gelatin content was increased to 15%w/w. JP chewable gels without (F5) or with menthol (F6) were prepared. Both formulations which provided satisfying texture, with no syneresis were further evaluated for stability testing.
The freshly prepared F5 and F6 chewable gels were wrapped in foil, vacuum-sealed, or kept as unwrapped tablets in a tightly closed glass bottle. Both formulations were stored at room temperature (27 ± 2 °C), 2–8 °C,40 °C for 1 month, or under thermal cycling. It had been found that keeping both F5 and F6 and their gel base at 40 °C and under thermal cycling exhibited slight deformation. Gelatin is generally formable at a set temperature of about 15 °C and melts at a temperature of approximately 25–40 °C. However, our result was different from the previous studies which reported that the chewable gelatin base was physically stable at 40 ± 2 °C for 90 days (Prakash et al., 2014). This may be due to various factors affecting the melting temperature, such as the gelatin concentration, pH, gel strength, and other ingredients (Osorio et al., 2007).
After storage of F5 and F6 chewable gels at room temperature and 2–8 °C for 1 month, their texture was evaluated by organoleptic testing and texture analyser. Texture characteristics of chewable gels performed with a texture analyser including hardness, springiness, cohesiveness, gumminess, and chewiness were analysed. Hardness is the force required to compress a chewable gel to attain a given deformation. Springiness is the rate and extent to which a deformed material recovers to its original shape after the force is removed. Cohesiveness is the strength of the internal bonds in the chewable gel which retains its form before it breaks. Gumminess is the energy required to break a chewable gel into fragments until it is ready to swallow. It is defined as hardness × cohesiveness. Chewiness is the time or work needed for masticating a chewable gel until it is ready for swallowing. It is defined as gumminess × springiness (Mousavi et al., 2019).
The results indicated that chewable gels F5 and F6 in foil-wrapped packages or bare tablets were not physically stable at room temperature (27 ± 2 °C) and 2–8 °C. More rigidity of both formulations in foil-wrapped packages may be due to some water loss from its structure resulting from the foil wrapping not being completely sealed. Moreover, foil-wrapped packaging formed some creases on the gel surface resulting unattractive product. However, an appropriate packaging to maintain their texture was a vacuum-sealed package; F5 was physically stable when stored at room temperature or in the refrigerator while F6 should be kept at room temperature. It has been noted that chewable gels kept in a vacuum sachet were slightly flattened, however, they could rather return to their original shape and size. Under the same determined storage conditions and packaging, chewable gel F5 and F6 were chemically stable for both pH and gallic acid content. A good manufacturing practice approach is applied for the development of these formulations; therefore, they are capable of resisting microbial contamination within a determined time.
However, organoleptic examinations such as smelling and tasting for F5 and F6 chewable gels under ethical approvals and real-time (long-term) stability studies are needed for further investigation.
CONCLUSION
JP chewable gels containing JP extract were successfully developed in the current investigation using gelatin, stevioside syrup, sorbitol solution, menthol, and paraben concentrate. Variations in the packaging (foil-wrapped, vacuum-sealed, unwrapped) did not impact the physical appearance, pH value, microbiological growth, or GA content of either JP chewable gel. For acceptable physical, chemical, and biological stability, it is recommended that JP chewable gels F5 and F6 are packed in vacuum-sealed packaging and stored at room temperature. Both formulations are easy to use and could increase patient or customer compliance when using JP as a remedy for various conditions such as hyperlipidaemia and weight management.
ACKNOWLEDGMENTS
The authors thank the Faculty of Pharmaceutical Sciences, Naresuan University for providing instruments.
AUTHOR CONTRIBUTIONS
Natthapat Saibuathong and Thanaphon Boonsirirungrueng assisted in conducting the experiments, performed the statistical analysis and data visualization. Pattana Sripalakit helped supervise the project. Aurasorn Saraphanchotiwitthaya designed and conducted all of the experiments, supervise the project and wrote the manuscript. All authors have read and approved of the final manuscript.
CONFLICT OF INTEREST
The authors declare that they hold no competing interests.
REFERENCES
Banjongsinsiri, P., Donrung, N., and Pasakawee, K. 2020. Effect of gelation addition on physico-chemical characteristics of bastard oleoaster gummy jelly. Proceedings of the 7th International Conference on Food Agriculture & Biotechnology 2020.
Chattopadhyay, S., Raychaudhuri, U., and Chakraborty, R. 2014. Artificial sweeteners - a review. Journal of Food Science and Technology. 51(4): 611–621.
Čižauskaite, U., Jakubaityte, G., Žitkevičius, V., and Kasparavičienė, G. 2019. Natural ingredients-based gummy bear composition designed according to texture analysis and sensory evaluation in vivo. Molecules. 24(7): 1442.
Davydova, N. 2018. USP chewable gels monographs. USP Dietary Supplements Stakeholder Forum. USA, Maryland.
Dille, M.J., Hattrem, M.N., and Draget, K.I. 2018. Soft, chewable gelatin-based pharmaceutical oral formulations: A technical approach. Pharmaceutical Development and Technology. 23: 504–511.
Gupta, R., Singhal, S., Goyle, A., and Sharma, V.N. 2001. Antioxidant and hypocholesterolaemic effects of Terminalia arjuna tree-bark powder: A randomised placebo-controlled trial. The Journal of the Association of Physicians of India. 49: 231–235.
Kardani, K., Gurav, N., Solanki, B., Patel, P., and Patel, B. 2013. RP-HPLC method development and validation of gallic acid in polyherbal tablet formulation. Journal of Applied Pharmaceutical Science. 3(5): 37–42.
Liu, F., Ranmal, S., Batchelor, H.K., Orlu-Gul, M., Ernest, T.B., Thomas IW, Flanagan, T., and Tuleu, C. 2014. Patient-centred pharmaceutical design to improve acceptability of medicines: similarities and differences in paediatric and geriatric populations. Drugs. 74(16): 1871–1889.
Miquel-Kergoat, S., Azais-Braesco, V., Burton-Freeman, B., and Hetherington, M.M. 2015. Effects of chewing on appetite, food intake and gut hormones: A systematic review and meta-analysis. Physiology & Behavior. 151: 88-96.
Morten D., Magnus, H., and Kurt, D. 2017. Soft, chewable gelatin-based pharmaceutical oral formulations: A technical approach. Pharmaceutical Development and Technology. 23: 1-28.
Mousavi, M., Heshmati, A., Daraei Garmakhany, A., Vahidinia, A., and Taheri, M. 2019. Texture and sensory characterization of functional yogurt supplemented with flaxseed during cold storage. Food Science Nutrition. 7(3): 907-917.
Osorio, F.A., Bilbao, E., Bustos, R., and Alvarez, F. 2007. Effects of concentration, bloom degree, and pH on gelatin melting and gelling temperatures using small amplitude oscillatory rheology. International Journal of Food Properties. 10(4): 841–851.
Pathompak, P., Charoenchai, L., and Monton, C. 2015. The cholesterol esterase inhibition and total phenolic content of aqueous extract of triphala and modified triphala formulas. Bulletin of Health, Science and Technology. 13(2): 25–30.
Patil, R.H., Prakash, K., and Maheshwari, V.L. 2011. Hypolipidemic effect of Terminalia arjuna in experimentally induced hypercholesteremic rats. Acta Biologica Szegediensis. 55(2): 289–293.
Polshettiwar, S.A., Sawant, D.H., Abhale, N.B., Chavan, N.B., Baheti, A.M., Wani, M.S., Tagalpallewar, A.A., Deshmukh, C.D., and Polshettiwar, A. 2022. Review on regulation of herbal products used as a medicine across the globe: a case study on turmeric - golden medicine. Biomedical and Pharmacology Journal. 15(3): 1227–1237.
Prakash, K., Satyanarayana, V.M., Nagiat, H.T., Fathi, A.H., Shanta, A.K., and Prameela, A.R. 2014. Formulation development and evaluation of novel oral jellies of carbamazepine using pectin, guar gum and gellan gum. Asian Journal of Pharmaceutical Sciences. 8(4): 241–249.
Rinthong, P., Mingmalairak, S., and Tantisira, M. 2016. Preclinical evaluation of lipid lowering effect and acute toxicity of Thai herbal formulary, chatuphalatika. The Thailand Research Fund and Mahasarakham University, Thailand. Report No.: TRG5780201.
Rodríguez-Pombo, L., Awad, A., Basit, A. W., Alvarez-Lorenzo, C., and Goyanes, A. 2022. Innovations in chewable formulations: The novelty and applications of 3D printing in drug product design. Pharmaceutics. 14(8): 1732.
Sabri, L.A., Khasraghi, A.H., and Sulaiman, H.T. 2022. Preparation and evaluation of oral soft chewable jelly containing flurbiprofen. Journal of Advanced Pharmaceutical Technology & Research. 13(4): 306-311.
Suwan, T., Pradutprom, W., Ngamroop, W., Choosuk, N., and Phungamngoen, C. 2017. Development of Babbler’s bill leaf gummy jelly. Burapha Science Journal. 22(1): 189–201.
Thakur, C.P., Thakur, B., Singh S, Sinha, P.K., and Sinha, S.K. 1988. The Ayurvedic medicines haritaki, amla and bahira reduce cholesterol-induced atherosclerosis in rabbits. International Journal of Cardiology. 21(2): 167–175.
Thilavech, T., Sutiyaporn, A., Kanchanadumkerng, P., Sato, V.H., Parichatikanond, W., Charoenwiwattanakij, P., and Chewchinda, S. 2023. Development of gummy jelly incorporated with Lysiphyllum strychnifolium leaf extract and its antioxidant and α-glucosidase inhibitory activities. Natural and Life Sciences Communications. 22(2): e2023019. https://doi.org/10.12982/NLSC.2023.019
Zhou, Q., Zhang, Z., Huang, Y., Niu, L., Miao, J., and Lai K. 2022. Effects of acidulants on the rheological properties of gelatin extracted from the skin of Tilapia (Oreochromis mossambicus). Foods. 11(18): 2812.
OPEN access freely available online
Natural and Life Sciences Communications
Chiang Mai University, Thailand. https://cmuj.cmu.ac.th
Aurasorn Saraphanchotiwitthaya1, 2,*, Natthapat Saibuathong1, Thanaphon Boonsirirungrueng1 and Pattana Sripalakit 2, 3
1 Department of Pharmaceutical Technology, Faculty of Pharmaceutical Sciences, Naresuan University, Phitsanulok 65000, Thailand.
2 Pharmaceutical Biotechnology Research Unit, Faculty of Pharmaceutical Sciences, Naresuan University, Phitsanulok 65000, Thailand.
3 Department of Pharmaceutical Chemistry and Pharmacognosy, Faculty of Pharmaceutical Sciences, Naresuan University, Phitsanulok 65000, Thailand.
Corresponding author: Aurasorn Saraphanchotiwitthaya E-mail: aurasorns@nu.ac.th
Total Article Views
Editor: Nisit Kittipongpatana,
Chiang Mai University, Thailand
Article history:
Received: April 19, 2022;
Revised: July 18, 2023;
Accepted: July 24, 2023;
Online First: August 18, 2023

