
Antioxidant, Antibacterial Activities and Cytotoxicity of Garlic Leaf Extract from Garlic Waste
Panee Sirisa-ard, Kiatisak Pholsonklam, Apinya Satchachai, Yingmanee Tragoolpua and Thida Kaewkod*Published Date : August 9, 2023
DOI : https://doi.org/10.12982/NLSC.2023.059
Journal Issues : Number 4, October-December 2023
Abstract Five garlic parts (root, clove skin, leaf, flower stalk and garlic peel) of garlic waste from garlic harvest processing were used in this study. Each part of the garlic waste was screened for total phenolic, flavonoid and antioxidant activity after extraction with 70% ethanol. Interestingly, garlic leaf (GLf) showed the greatest richness of total phenolic, flavonoid and antioxidant activity. GLf was selected to extract with water and 70% ethanol and the highest extraction yield was achieved with water extract. The GLf extracts demonstrated a good quality standard of physical and chemical properties. The aqueous extract of GLf revealed the highest total phenolic content of 1,676.40 ± 18.02 mg GAE/100 g extract. Both aqueous and ethanolic extracts did not differ regarding total flavonoid contents, showing values of 118.60 ± 6.10 mg QUE/100 g extract and 110.90 ± 2.30 mg QUE/100 g extract, respectively. Phenolic compounds including gallic acid, catechin and tannic acid were detected in the aqueous and ethanolic extracts of GLf by HPLC assay. Moreover, four flavonoid compounds including isoquercetin, quercetin, kaempferol and rutin were detected in aqueous extract whereas the ethanolic extract presented apigenin, isoquercetin, quercetin and kaempferol. The ethanolic extract of GLf demonstrated the stronger antioxidant activity than aqueous extract. GLf extracts also exhibited antimicrobial activity against pathogenic bacteria with MIC and MBC values of 125-250 mg/mL. In addition, GLf aqueous extract showed lower toxicity on Vero cells than ethanolic extract. Therefore, GLf waste can be used as a cheap source of natural compounds and antioxidants, with potential applications in different biological, medicinal and food products.
Keywords: Allium sativum L., Antibacteria, Antioxidant, Garlic, Toxicity
Citation: Sirisa-ard, P., Pholsonklam, K., Satchachai, A., Tragoolpua, Y., and Kaewkod, T. 2023. Antioxidant, antibacterial activities and cytotoxicity of garlic leaf extract from garlic waste. Natural and Life Sciences Communications. 22(4): e2023059.
INTRODUCTION
Allium sativum L. (garlic) is classified in the family Amaryllidaceae. It originated in Asia and widely cultivated in Egypt, Mexico, China and Europe (Subroto et al., 2021). Garlic has been used throughout history for both culinary and medicinal purposes. Presently, garlic is grown almost everywhere and is known to have more than 300 varieties (Majewski, 2014). The parts of A. sativum including roots (GRt), bulbs (Gb), cloves (Gc), leaves (GLf), stems (GStm) and flowers (Gf) are used to prepare mixtures and decoctions to manipulate various ailments (Qiu et al., 2022). A. sativum, the perennial herbaceous plant (Figure 1), is large with upright flowering stems that extends up to 1 m. The garlic leaf blades are linear, flat, robust and approximately 0.5 to 1.0 in (1.25 to 2.5 cm) wide, with a pointed apex and flowers are violet to fuchsia. Slender leaves on the exterior of the odoriferous bulbs surround an internal sheath containing the cloves, and each bulbs contains 10 to 20 cloves (Londhe et al., 2011; Meriga et al., 2012).
Garlic is a particularly rich source of organosulfur compounds such as allicin, which are partly responsible for health beneficial effects. Other phytochemical compounds found in garlic including diallyl disulphide (DDS), diallyl trisulfide (DTS) and S-allyl cysteine (SAC) have a variety of pharmacologic properties (Subroto et al., 2021). Garlic compounds have been reported as promising immune boosters and used for treatment of various diseases such as cardiovascular ailments, neoplastic growth, rheumatism, diabetes, intestinal worms, flatulence, colic, dysentery, liver diseases, facial paralysis, tuberculosis, bronchitis, high blood pressure and several other diseases (Jikihara et al., 2015; Naji et al., 2017; Morihara et al., 2017; Takashima et al., 2017; Tudu et al., 2022). In Thailand, many foods are produced using garlic cloves as an ingredient.
Garlic is widely cultivated in northern and northeastern Thailand that could produce around 75,444 ton yearly (Maufaey et al., 2022). Moreover, the processing of garlic cloves yields a considerable amount of waste products by 35% (w/w). Garlic wastes such as leaf blades (GLf), flower stalks (GSt), garlic peels (Gp) and roots (GRt) obtained from garlic harvest process (Figure 1B) have been used as bioresource, feed alimentation, soil amendment and may be used as a potential source of bioactive components (Kallel and Chaabouni, 2017). Garlic stem and leaf blades wastes were found to contain allicin, which is the major bioactive component of garlic. The allicin in garlic stem and leaf blades was lower than that found in bulbs (Mohsen and Shahab, 2010). However, information about their biochemical compounds such as total phenolic content especially flavonoids and their bioactivity in other waste parts have not been studied.
Flavonoids, a group of natural substances with variable phenolic structures, are well known for their beneficial health effects. Flavonoids are now considered as an indispensable component in a variety of nutraceutical, pharmaceutical, medicinal and cosmetic applications (Petkova et al., 2023). This is attributed to their antioxidative, antiinflammatory, antimutagenic and anticarcinogenic properties coupled with their capacity to modulate key cellular enzyme functions (Panche et al., 2006; Khelfi et al., 2023; Warinthip et al., 2023). Quercetin, a flavonoid commonly presents in fruits and vegetables including garlic possesses. It also demonstrates antiviral properties (Sharma, 2019). In addition, other flavonoids such as caffeic acid, ferulic acid, vanillic, p-hydroxybenzoic, p-coumaric acids and sinapic acid are also found in garlic (Beato et al., 2011). Therefore, this work aimed to study the phytochemical compounds especially flavonoids and phenolics in garlic waste extracts. Antioxidant, antibacterial and cytotoxic activities of these extracts were also investigated. Moreover, the biological activities of garlic waste extracts from this study could be promoted as potential raw materials for drugs to prevent chronic diseases and future research directions.

Figure 1. Different parts of Allium sativum L. (A, modified from Rader and McGuinnes, 2022) and waste parts from the garlic harvest process (B)
MATERIALS AND METHODS
Raw materials
The dry garlic wastes were separated in five parts according to the processing of garlic wastes from the fleshy cloves (Gc) including roots (GRt), clove skin (GSk), leaf (GLf), woody flower stalk and undeveloped leaf blade (GSt), and garlic peel (Gp). All parts of dried garlic wastes were supplied by Northern Siam Seedlac Co., Ltd., Lampang Province, Thailand and collected from northern Thailand in February 2020. All garlic parts were cleaned and dried using a hot air oven at 40oC for 48 hours. Then, the garlic parts were checked for their moisture content to be in the range of 10-12% before crushing passed through a number 40 mesh sieve.
Phytochemical screening of garlic wastes
All parts of dried garlic wastes including GRt, GSk, GLf, GSt and Gp were coarsely ground using a blender. The garlic waste powders were extracted by maceration method using 70% (v/v) ethanol as a solvent at a ratio of sample and solvent equal to 1:5 (w/v) and incubated at room temperature under shaking for 48 hours (Abubakar, 2009). Additionally, Gc was also extracted as a control and compared with garlic wastes. The extracts were filtered using Whatman No. 1 paper and the residues were extracted with the solvent again. The extract filtrate was then evaporated using a rotary evaporator at 50°C under reduced pressure 105 to 70 lb. Therefore, the extracts were screened for phytochemical compositions including total phenolic and flavonoid contents and antioxidant activity.
Extraction of selected garlic waste
The highest phytochemical compositions of garlic wastes were selected to extract by Soxhlet extraction (Chhouk et al., 2017). The dried garlic waste was extracted with two extraction methods using ethanol and water. The ethanol extraction was processed with 70% (v/v) ethanol by Soxhlet apparatus at a temperature of 70°C for 24 hours. The extracts were filtered using Whatman No. 1 paper and the extract was then evaporated using a rotary evaporator at 50°C under reduced pressure 105 to 70 lb. Aqueous extract of garlic waste was prepared by boiling 2 kgs of dry garlic waste with a high speed extractor (Euro Best Technology Co., Ltd., model HXL-5E2) to make a 1o Brix of solution or 65.6 kgs and evaporated to 5o Brix or 9 kgs. Maltodextrin, 5% or 450 gms was added to obtain a 9o Brix. After that, the extracts were dried by spray dry technique using a spray dryer (Euro Best Technology Co., Ltd., model SDE-5) with inlet temperature of 160℃ and outlet temperature of 90°C, under 1.5 bar pressure and the speed of blower was 2,300 rpm/min. Then, the extract yields were calculated.
Physical and chemical properties of garlic waste
The physical and chemical properties of garlic waste were investigated concerning its quality following the Thai Herbal Pharmacopoeia (THP) standard (Thai Herbal Pharmacopoeia, 2022) including moisture content, color, pH, % Brix, specific gravity, alcohol extractive value, total ash value, acid insoluble ash, saturated chloroform aqueous extractive value, petroleum ether extractive value and chloroform extractive value.
Determining total phenolic content (TPC)
TPC of samples was examined using the Folin-Ciocalteau colorimetric method (Stratil et al., 2006). The sample solutions (1 mL) were mixed with 5 mL of 10% Folin-Ciocalteau reagent. The Folin-Ciocalteau reagent was diluted with distilled water at a ratio of 1:10 (v/v) before use. After incubating for 8 min, 4 mL of the 7.5% sodium carbonate solution was added, and the reaction was incubated in the dark at room temperature for 2 hours. Finally, the absorbance of the test samples was measured at 765 nm using a spectrophotometer (Milton Roy Spectronic 21D, Artisan Technology Group, IL, USA). The total phenolic content was calculated as natural compound (gallic acid) equivalent (GAE) by the following equation: Y = 0.0144X + 0.0017, and expressed as mg gallic acid equivalent per one hundred gram of extract (mg GAE/100 g extract).
Determining total flavonoid content (TFC)
The TFC of samples was examined using aluminum chloride colorimetric method modification (Djeridane et al., 2006). The sample (1 mL) was placed in a volumetric flask and 4 mL of distilled water was added followed by 0.5 mL of 5% sodium nitrite solution. After incubating at room temperature for 5 min, 0.5% aluminum chloride solution was added to the reaction and incubated at room temperature for 6 min. In addition, 2 mL of 1 M sodium hydroxide solution was added, and the volume was made up to 10 mL with distilled water. The solution was mixed thoroughly, and the absorbance was measured at 510 nm using a spectrophotometer (Milton Roy Spectronic 21D, Artisan Technology Group, IL, USA). The total flavonoid content was calculated as natural compound (quercetin) equivalent (QUE) by the following equation: Y = 0.01X + 0.0097, and expressed as mg quercetin equivalent per one hundred gram of extract (mg QUE/100 g extract).
High performance liquid chromatography (HPLC)
The four phenolic compounds, gallic acid, hydroquinone, catechin and tannic acid, and six flavonoid compounds, eriodictyol, apigenin, isoquercetin, quercetin, kaempferol and rutin in garlic wastes were detected using HPLC (Penarrieta et al., 2007). The extracts and the standard compounds were filtered through a 0.45 µm microfilter and 20 µL of the filtered sample was injected in the HPLC system (Agilent Technologies, Series 1,100 liquid chromatograph (Germany) with a mass detector system, Agilent Technologies LC/MED SL (USA). Separation was achieved using a reversed-phase LiChroCART RP-18e (4.6 x 150 mm, 5 µm, Purosher STAR MERCK, USA). The mobile phase consisted of mobile phase A: acetonitrile and mobile phase B: 10 mM ammonium formate buffer, pH 4 with formic acid. The gradient elution was shown as follows: 0 to 5 min 100% B constant, 5 to 10 min A 0% to 20%, 10 to 20 min A 20% constant and 20 to 60 min A 20 to 40%, and zero-time condition was adjusted in mobile phase A at 8% and continuously increased to 78% for 50 min. The chromatographic column was maintained at 40°C and the flow rate was used at 1.0 mL/min. Eluted peaks were detected at 280, 360 and 530 nm. The amount of quercetin in the extracts was calculated by comparing with the standard for the compound.
Antioxidant activity
The extract was investigated for antioxidant activity using the 2,2–diphenyl–1–picrylhydrazyl (DPPH) radical scavenging assay (Wojdylo et al., 2007). A solution of 81.2 mM DPPH radicals was prepared in methanol, and the sample solution (1 mL) was mixed with 5 mL of DPPH solution. The mixtures were vigorously shaken and incubated in the dark at room temperature for 30 min. The absorbance was measured using a spectrophotometer at 517 nm. Methanol was used as a blank control, while DPPH without the extract was used as a radical control. The DPPH radical scavenging activity was calculated and expressed as the percentages of DPPH free radical scavenging activity as described below.
Percentages of DPPH free radical scavenging activity (%) = [(Ao-A1)/Ao] × 100
Where Ao was the absorbance of the DPPH solution and A1 was the absorbance of the sample mixed with the DPPH solution. The concentrations providing 50% scavenging (IC50) were calculated from the graph plotted between the free radical scavenging percentages and the sample concentrations.
Antibacterial activity
The antibacterial activity of garlic waste was determined by minimal inhibitory concentration (MIC) and minimal bactericidal concentration (MBC) using the broth dilution method (CLSI, 2018). A single colony of tested pathogenic bacteria including Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus, Proteus vulgaris, Salmonella Typhi, Klebsiaella pneumoniae and Streptococcus pyogenes were inoculated in Mueller-Hinton broth (MHB) and incubated at 37°C for 18 to 24 hours. The garlic waste was dissolved with dimethyl sulfoxide (DMSO) at a concentration of 500 mg/mL as a stock solution. The MHB (0.1 mL) was dispensed in a sterile 96-well plate. The stock solution of the extract (0.1 mL) was added to the first test well and the extract was serial two-fold diluted in each well in the 96-well plate. The bacterial culture (McFarland standards No.0.5) was added to all wells. The mixture of extract and bacterial culture were incubated at 37°C for 18 to 24 hours. The MIC was determined from the lowest concentration of the extract that did not show any bacterial growth. Therefore, MBC value was determined from each well showing no visible bacteria growth of as compared with the bacterial growth control. The culture broth not displaying bacterial growth was streaked on Mueller-Hinton agar (MHA), and bacteria colonies were determined after incubation. The MBC value was the lowest concentration that inhibiting bacterial growth by 99.9%.
Cytotoxicity test of garlic waste on Vero cells
The cytotoxicity of the extract on Vero cells was tested using the MTT assay (Umthong et al., 2011). Vero cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM; Gibco, Grand Island, NY, USA) supplemented with 10% heat-inactivated fetal bovine serum (HyCloneTM, Pittsburgh, PA, USA), 100 units/mL penicillin, and 100 μg/mL streptomycin (CAISSON, Smithfield, UT, USA). After incubating at 37°C in a 5% CO2 incubator (SHEL LAB, Cornelius, OR, USA), the cells were washed twice with phosphate buffer saline (PBS, pH 7.4) and trypsinized with 0.05% trypsin-EDTA solution (CAISSON, USA). The cells were harvested by centrifugation at 1,200 rpm, 4°C for 5 min. Then the cells were resuspended with medium and counted using a hemacytometer to adjust the number of cells. The cell numbers of 105 cell/mL were plated in 96-well plates and incubated at 37°C in a 5%CO2 incubator for 24 hours. After incubating, each concentration of the extract was treated with cells and incubated at 37°C in a 5%CO2 incubator for 48 hours. Then the MTT solution (Bio Basic Inc., Markham, ON, Canada) was added and incubated for 4 hours. Finally, the blue formazan crystal was dissolved using dimethyl sulfoxide and absorbance was measured at 540 and 630 nm. The percentage of cell viability and 50% inhibitory concentration (IC50) were calculated and compared with the control cell.
Statistical analysis
All experiments were performed in three independent treatments. All data acquired from the treatments and the control groups were compared and analyzed and were presented as mean ± SD using the t-test and ANOVA analysis.
RESULTS
Phytochemical screening of garlic wastes
Each garlic waste part including the fleshy cloves (Gc), roots (GRt), clove skin (GSk), leaf (GLf), roots (GRt), clove skin (GSk), flower stalks (GSt), and garlic peels (Gp) were screened for phenolic, flavonoid content and antioxidant capacity. The results indicated that all garlic wastes showed quantities of phenolics, flavonoid, and antioxidant capacities (Table 1). Interestingly, GLf extract showed the highest contents of phenolics and flavonoids with values of 861.07 ± 7.13 mg GAE/100 g extract and 110.90 ± 2.30 mg QUE/100 g extract that was higher than the common use of Gc and the other garlic waste parts (Table 1). This was followed by the GRt extract, containing the phenolic and flavonoid contents of 289.00 ± 3.06 mg GAE/100 g extract and 28.20 ± 0.85 mg QUE/100 g extract, respectively. For antioxidant activity, GLf extract also presented the greatest antioxidant capacities with an IC50 of 43.77 ± 0.72 mg/mL. The GRt extract represented the antioxidant activity with an IC50 of 68.26 ± 0.60 mg/mL (Table 1). Therefore, the GLf extract was further used to investigate and identify phytochemical compounds, antibacterial activity and cytotoxicity.
Table 1. Content of phenolics, flavonoids and antioxidant activity from garlic waste extracts.
|
Garlic waste extract |
Total phenolic content (mg GAE/100 g extract) |
Total flavonoid content (mg QUE/100 g extract) |
Antioxidant activity (IC50, mg/mL) |
|
Roots (GRt) |
289.00 ± 3.06 |
28.20 ± 0.85 |
68.26 ± 0.60 |
|
Flower stalks (GSt) |
49.81 ± 1.34 |
10.32 ± 0.39 |
369.15 ± 9.06 |
|
Peels (Gp) |
79.52 ± 3.06 |
4.43 ± 1.21 |
223.26 ± 2.41 |
|
Leaf (GLf) |
861.07 ± 7.13* |
110.90 ± 2.30* |
43.77 ± 0.72* |
|
Clove skin (GSk) |
164.60 ± 2.44 |
18.95 ± 0.54 |
78.54 ± 1.39 |
|
Control fleshy cloves (Gc) |
16.98 ± 0.28 |
4.43 ± 1.99 |
132.47 ± 3.12 |
Note: The data represents mean values of three replicates ± SD. *The statistical data show the highest value in each assay (P <0.05).
Physical and chemical properties of garlic waste
The GLf showed high amount of phenolics, flavonoids and antioxidant capacity that was selected to analyze physical and chemical properties. The physical and chemical examinations included moisture content, color, pH, % Brix, specific gravity, alcohol extractive value, total ash value, acid insoluble ash, saturated chloroform aqueous extractive value, petroleum ether extractive value and chloroform extractive value. After extraction GLf with 70% ethanol, the crude extract showed a percentage yield of 18.21% and represented in dark green. In addition, the pH, % Brix and specific gravity of GLf extract were 6.3167, 19%, and 1.0341, respectively. The standard value of quality control of GLf extract indicated that the percentage of moisture content was 9.72 ± 0.0027% (Table 2). The amount of total ash and acid-insoluble ash of GLf extract were 14.2481 and 0.8633%. Moreover, the extractive value in different solvents including alcohol, petroleum ether, chloroform and saturated chloroform aqueous demonstrated that GLf extract showed the highest percentage of yield after extraction by saturated chloroform aqueous, with a value of 27.0494% (Table 2).
Table 2. Physical and chemical properties of garlic leaf waste.
|
No. |
Measurement |
Value |
Standard value |
|
1 |
Yield |
18.21% (w/w) |
Continuous extraction with 70% ethanol |
|
2 |
Color |
Dark green |
Not Available |
|
3 |
pH |
6.3167 ± 0.006 |
Not Available |
|
4 |
% Brix |
19 ± 0 % Brix |
Not Available |
|
5 |
Specific gravity |
1.0341 ± 0.051 |
Not Available |
|
6 |
Moisture content |
9.72 ± 0.0027% (w/w) |
≤ 10% (w/w) |
|
7 |
Total ash |
14.2481 ± 0.24% (w/w) |
≤ 14% (w/w) |
|
8 |
Acid-insoluble ash |
0.8633 ± 0.19% (w/w) |
≤ 5% (w/w) |
|
9 |
Alcohol extractive value |
3.6019 ± 0.13 % (w/w) |
Not Available |
|
10 |
Petroleum ether extractive value |
2.6533 ± 0.08% (w/w) |
Not Available |
|
11 |
Chloroform extractive value |
3.5733 ± 0.15% (w/w) |
Not Available |
|
12 |
Saturated chloroform aqueous extractive value |
27.0494 ± 0.93% (w/w) |
Not Available |
Note: The data represent mean values of three replicates ± SD. a, b indicating significant difference (P <0.05).
Total phenolic content (TPC) and total flavonoid content (TFC) of garlic leaf (GLf) extracts
The GLf waste was selected to extract with 70% ethanol and water, and their properties was compared. After extracting, the ethanolic and aqueous extracts exhibited percentage yields of 18.21% (w/w) and 21.80% (w/w), respectively. The TPC and TFC of GLf extracts are shown in Table 3. The TPC of the extracts were expressed as gallic acid equivalents, mg GAE/100 g extract. The TPC of the aqueous GLf extract was significantly higher than the ethanolic extract. The aqueous extract of GLf revealed the highest TPC of 1,676.40 ± 18.02 mg GAE/100 g extract, whereas the ethanolic extract was 861.07 ± 7.13 mg GAE/100 g extract. Regarding the TFC, both aqueous and ethanolic extracts did not differ in the TFC, showing values of 118.60 ± 6.10 mg QUE/100 g extract and 110.90 ± 2.30 mg QUE/100 g extract, respectively.
Table 3. Total phenolic and flavonoid contents of garlic leaf extracts.
|
Garlic leaf extract |
Total phenolic content |
Total flavonoid content |
|
Aqueous extract |
1,676.40 ± 18.02 a |
118.60 ± 6.10 a |
|
Ethanolic extract |
861.07 ± 7.13 b |
110.90 ± 2.30 a |
Note: The data represent mean values of three replicates ± SD. a, b indicating significant difference (P <0.05).
Phytochemical analysis of garlic leaf (GLf) extracts using HPLC
The four phenolic compounds; gallic acid, hydroquinone, catechin and tannic acid, and six flavonoid compounds; eriodictyol, apigenin, isoquercetin, quercetin, kaempferol and rutin in GLf extracts were detected using the HPLC assay. The result showed that the aqueous and ethanolic extracts of GLf exhibited differences concerning phenolic and flavonoid compounds (Table 4). The aqueous and ethanolic extracts of GLf revealed three phenolic compounds such as gallic acid, catechin and tannic acid. The tannic acid showed the highest content in aqueous extract of 130.25 mg/kg of extract, while gallic acid exhibited a high content in ethanolic extract of 212.61 mg/kg of extract (Table 4). Regarding flavonoid compounds, the aqueous extract of GLf detected four flavonoid compounds including isoquercetin, quercetin, kaempferol and rutin, whereas the ethanolic extract represented apigenin, isoquercetin, quercetin and kaempferol. Rutin showed the highest content in aqueous extract of 88.45 mg/kg of extract. Moreover, apigenin and quercetin showed a high content in ethanolic extract with values of 63.37 and 68.38 mg/kg of extract, respectively (Table 4).
Table 4. Phytochemical compound contents in garlic leaf extracts using HPLC.
|
No. |
Compounds |
Phytochemical compound contents (mg/kg extract) |
|
|
Aqueous extract |
Ethanolic extract |
||
|
Phenolic compound |
|||
|
1 |
Gallic acid |
87.40 |
212.61 |
|
2 |
Hydroquinone |
Undetected |
Undetected |
|
3 |
Catechin |
33.34 |
104.22 |
|
4 |
Tannic acid |
130.25 |
112.64 |
|
Flavonoid compound |
|||
|
5 |
Eriodictyol |
Undetected |
Undetected |
|
6 |
Apigenin |
Undetected |
63.37 |
|
7 |
Isoquercetin |
46.47 |
43.37 |
|
8 |
Quercetin |
39.54 |
68.38 |
|
9 |
Kaempferol |
<10 |
0.30 |
|
10 |
Rutin |
88.45 |
Undetected |
Antioxidant activity of garlic leaf (GLf) extracts
The DPPH radical scavenging activities of GLf extracts are presented in Table 5. The results showed that the ethanolic extract of GLf demonstrated the strongest antioxidant activity with IC50 of 43.77 ± 0.72 mg/mL. Moreover, the aqueous extract of GLf exhibited the ability to inhibit DPPH radicals with the IC50 value of 87.63 ± 10.00 mg/mL.
Table 5. Antioxidant capacities of garlic leaf extracts.
|
Garlic leaf extract |
Antioxidant capacity, IC50 (mg/mL) |
|
Aqueous extract |
87.63 ± 10.00b |
|
Ethanolic extract |
43.77 ± 0.72a |
Note: Data represent mean values of three replicates ± SD. IC50: half-maximal inhibitory concentration. a, b indicate significant difference (P < 0.05).
Antibacterial activity of garlic leaf (GLf) extracts
The efficacy of GLf extracts was assessed for antibacterial activity on pathogenic bacteria: Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus, Proteus vulgaris, Salmonella Typhi, Klebsiella pneumoniae and Streptococcus pyogenes. In this study, the inhibitory activities of GLf extracted by water and ethanol were investigated for the MIC and MBC against pathogenic bacteria using the broth dilution method. The result demonstrated that the aqueous and ethanolic extracts of GLf revealed the inhibitory effect on all tested bacteria. Moreover, GLf extracts showed the lowest MIC and MBC values of 125 mg/mL against P. vulgaris, followed by E. coli, P. aeruginosa, S. aureus, S. Typhi, K. pneumoniae and S. pyogenes with the MIC and MBC values of 250 mg/mL (Table 6).
Table 6. MIC and MBC values of garlic leaf extracts against pathogenic bacteria.
|
Pathogenic bacteria |
Concentration of garlic leaf extract (mg/mL) |
|||
|
Aqueous extract |
Ethanolic extract |
|||
|
MIC |
MBC |
MIC |
MBC |
|
|
E. coli |
250 ± 0.0 |
250 ± 0.0 |
250 ± 0.0 |
250 ± 0.0 |
|
P. aeruginosa |
250 ± 0.0 |
250 ± 0.0 |
250 ± 0.0 |
250 ± 0.0 |
|
S. aureus |
250 ± 0.0 |
250 ± 0.0 |
250 ± 0.0 |
250 ± 0.0 |
|
P. vulgaris |
125 ± 0.0 |
125 ± 0.0 |
125 ± 0.0 |
125 ± 0.0 |
|
S. Typhi |
250 ± 0.0 |
250 ± 0.0 |
250 ± 0.0 |
250 ± 0.0 |
|
K. pneumoniae |
250 ± 0.0 |
250 ± 0.0 |
250 ± 0.0 |
250 ± 0.0 |
|
S. pyogenes |
250 ± 0.0 |
250 ± 0.0 |
250 ± 0.0 |
250 ± 0.0 |
Note: Data represents mean values of three replicates ± SD
Cytotoxicity of garlic leaf (GLf) extracts on Vero cells by MTT assay
The cytotoxicity of GLf extracts on Vero cells was determined for the toxicity of various concentrations of samples for 48 hours. The ethanolic and aqueous extracts of GLf showed the cytotoxicity on Vero cells by a dose-dependent manner (Figure 2). The ethanolic extract of GLf at concentrations of 0.020 – 2.500 mg/mL had ability to decrease the viability of Vero cells from 0.60 ± 0.29% to 96.88 ± 1.06%. In addition, the aqueous extract of GLf at concentrations of 0.391 – 25.000 mg/mL presented the toxicity on Vero cells ranged from 10.56 ± 1.67% to 97.08 ± 8.49%. Thus, the 50% cytotoxic dose (CD50) of GLf extracts was calculated for comparing to the toxicities of samples after treatment on Vero cells. The ethanolic and aqueous extracts of GLf presented the CD50 values against Vero cells of 0.868 ± 0.046 and 9.628 ± 0.036 mg/mL, respectively (Table 7). The result showed that the lowest CD50 of GLf ethanolic extract had the toxicity on the Vero cells higher than the aqueous extract of GLf (Figure 2 and Table 7).
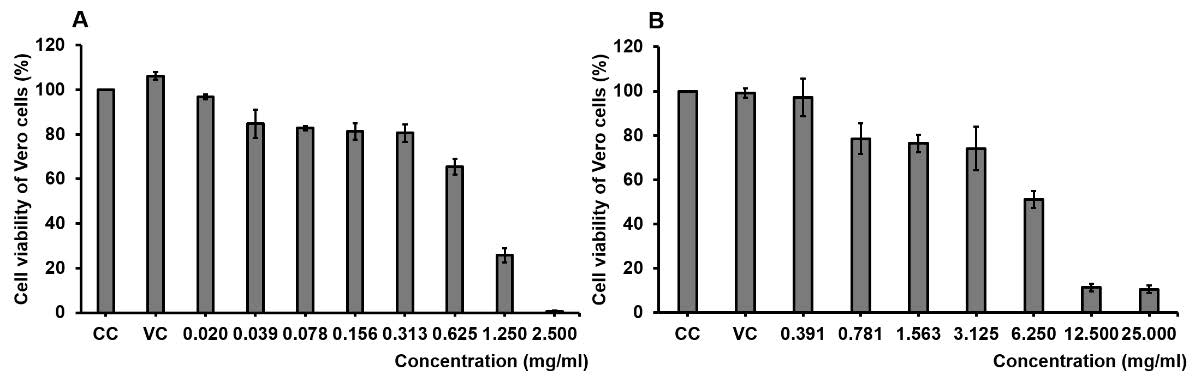
Figure 2. Cytotoxicity of Vero cells after treating with ethanolic (A) and aqueous (B) extracts of garlic leaf for 48 hours. Cell viability was measured using the MTT assay and calculated to compare with the control cells. Control (CC) and vehicle control (VC) cells were also treated with DMEM and 0.1% DMSO, respectively. The results are presented as mean ± SD of triplicate independent experiments.
Table 7. The 50% cytotoxic dose (CD50) of garlic leaf extracts after treating Vero cells for 48 hours.
|
Garlic leaf extracts |
50% Cytotoxic dose (CD50; mg/mL) |
|
Ethanolic extract |
0.868 ± 0.046a |
|
Aqueous extract |
9.628 ± 0.036b |
Note: Data represent mean values of three replicates ± SD. a, b indicating significant difference (P <0.05).
DISCUSSION
Allium sativum L., commonly called garlic, is a widely cultivated as not only foodstuffs but also medicinal plants globally since ancient times. Garlic contains many parts after the harvest processing such as leaf blades (GLf), flower stalks (GSt), bulbs (Gb) and roots (GRt). The most commonly used part is the Gb including the fleshy cloves (Gc) inside (Rader and McGuinnes, 2022). The Gc has been used as food ingredients and traditional medicines (Kallel and Chaabouni, 2017; Thomson and Ali, 2003). However, the garlic harvest processing produces garlic wastes such as GLf, GSt, garlic peels (Gp), and GRt that may contain as a potential source of beneficial substances. In this study, the garlic wastes including GRt, clove skin (GSk), GLf, GSt, and Gp were investigated for their phytochemical compounds, especially flavonoids and phenolics and biological activities such as antioxidant, anti-bacteria and toxicity on Vero cells. Each garlic waste part was extracted with 70% ethanol before phytochemical screening of total phenolic and flavonoid compounds, as well as antioxidant activity. The results showed that GLf extract revealed the highest contents of phenolics and flavonoids higher than the common use of Gc and the other garlic waste parts. In addition, GLf extract also showed antioxidant activity by scavenging DPPH radicals. GStm and GLf possess allicin, the major bioactive component of garlic, which is comparatively lower than that found in the Gb (Mohsen and Shahab, 2010). The GLf has been investigated to assess the antioxidant capacities and anti-cancer activities against HT-29 colorectal adenocarcinoma, HCT 116 colorectal carcinoma, HCT-8 [HRT-18] ileocecal colorectal adenocarcinoma and Ramos.2G6.4C10 Burkitt’s lymphoma cell lines (Liu et al., 2021). Moreover, GLf has also been reported to possess medicinal properties in various folk medicines and is used to treat various disease conditions like whooping cough (Bayan et al., 2014), asthma (Tesfaye and Mengesha, 2015), rheumatoid arthritis (Osbole et al., 2010), and hemorrhoids (Soladoye et al., 2010) in single or compound formulations. In India, natives of Arunachal Pradesh state use GLf, Gc, and GRt of A. sativum to treat cold, cough and skin rashes (Devi et al., 2014). Moreover, the GLf extract was quality determined regarding physical and chemical properties according to Thai Herbal Pharmacopoeia (THP) standard (Thai Herbal Pharmacopoeia, 2022), moisture content, color, pH, % Brix, specific gravity, alcohol extractive value, total ash value, acid insoluble ash, saturated chloroform aqueous extractive value, petroleum ether extractive value and chloroform extractive value. The total ash and acid-insoluble ash values were found to be 14.2481% (w/w) and 0.8633% (w/w) that included in the range of qualitative standards. Total ash is the residue remaining after incineration while acid insoluble ash is that part of total ash, which is insoluble in oil. HCl and water-soluble ash is that part of total ash which is soluble in water. Moreover, the purity and quality of crude drugs can be assessed using ash values (Gupta et al., 2014; Sharma et al., 2019). The moisture content of crude extract (9.72% w/w) was given below 10% (w/w). The moisture content should be minimum, as high moisture content leads to degrading the active constituents present in the plant material (Mandal et al., 2017). A study was conducted concerning alcohol, petroleum ether, chloroform, saturated chloroform aqueous extractive values and the results obtained were 3.6019% (w/w), 2.6533% (w/w), 3.5733% (w/w), and 27.0494% (w/w), respectively. This result shows that more saturated chloroform aqueous compounds are present in the chosen plant material. The extractive values of plant samples are useful to assess the phytochemical constituents present in the crude drug sample and help in approximating the solubility of constituents in a particular solvent (Mishra et al., 2011; Sharma et al., 2019).
According to the phytochemical compounds in GLf extracts, both aqueous and ethanolic extracts contained total phenolic and flavonoid compounds, especially the aqueous extract showed the highest content of phenolics (1,676.40 ± 18.02 mg GAE/100 g extract). This result was higher than the phenolic content of 297 mg GAE/100 g extract that found in the aqueous extract of garlic husk (Kallel et al., 2014). In addition, HPLC results demonstrated that three phenolic compounds including gallic acid, catechin and tannic acid were detected in aqueous and ethanolic extracts of GLf. The highest content of tannic acid was revealed from the aqueous extract, while gallic acid was shown as a high content in the ethanolic extract. For flavonoid compounds, four flavonoid compounds including isoquercetin, quercetin, kaempferol and rutin were detected in the aqueous extract of GLf whereas the ethanolic represented apigenin, isoquercetin, quercetin and kaempferol. The highest content of rutin was shown in the aqueous extract. Moreover, apigenin and quercetin showed a high content in the ethanolic extract. Hence, GLf extracts exhibited rich antioxidant activity related to these compounds. Several studies reported organosulfur compounds in GLf such as diallyl thiosulfinate, diallyl tetrasulfide, methyl allyl trisulfide, dipropyl sulfide, dially trisulfide, S-allyl cysteine sulfoxide, allyl methane sulfinate, diallyl disulfide, 3-vinyl-4H-1,2-dithiin, allyl methyl thiosulfinate, S-methyl cysteinesulfoxide, dipropyldi sulfide, dimethyl disulfide, 3-vinyl-6H-1,2-dithiin, methylpropyl disulfide, S-propyl cysteine sulfoxide, dimethyl sulfide, methyl methane sulfinate and 3-vinyl-6H-1,3-dithiin contributed to promote antioxidant and anticancer properties (Thomson and Ali, 2003; Fukaya et al., 2020).
Therefore, the aqueous and ethanolic extracts of GLf showed a strong antimicrobial agent on both Gram-positive and Gram-negative bacteria such as Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus, Proteus vulgaris, Salmonella Typhi, Klebsiella pneumoniae and Streptococcus pyogenes. In addition, GLf extracts demonstrated a high content of phenolics and flavonoids that also exhibited antimicrobial activity. Phenolic compounds had the ability to induce irreversible damage to the cytoplasmic membrane and inhibit intracellular enzymes, as they caused leakage of essential intracellular substances (Borges et al., 2013). Flavonoids have been shown to locate soluble proteins outside the bacteria cell walls; and thus, disrupt the formation of cell complexes (Cushnie and Lamb, 2011). Moreover, flavonoids may affect by inhibiting both energy metabolism and DNA synthesis; and thus, subsequent synthesis of RNA and proteins (Kumar and Pandey, 2013; Likittrakulwong et al., 2023). Quercetin and kaempferol are grouped as flavonoids and further studies have shown that they could decrease the ATPase activity of DNA helicase on K. pneumoniae and S. aureus (Chen and Huang, 2011; Huang et al., 2015). Similarly, the study of Abiy and Berhe (2016) demonstrated that the crude extract of Gb could inhibit clinical and standard isolates of S. aureus and E. coli. The GLf and Gb fractioned by methanol, ethyl acetate and chloroform showed antimicrobial activity against Bacillus subtilis and Aspergillus niger (Gunda et al., 2018). This fact contributed to several researchers who have discovered allicin (diallyl thiosulphinate) as main active component of garlic (Singh and Singh, 2019). The formation of allicin is followed by its rapid decomposition of sulphur-derived compounds so that the antimicrobial activity may be resulted from allicin (Harris et al., 2001).
This study also determined the cytotoxicity of GLf extracts on Vero cells as a normal cell line. The result revealed that ethanolic extract (CD50, 0.868 ± 0.046 mg/mL) had a toxicity on Vero cells higher than that of the aqueous extract (CD50, 9.628 ± 0.036 mg/mL) of GLf. However, the consumption of garlic has been recommended that garlic at high doses has the potential ability to induce liver damage, but low doses (0.1 or 0.25 g/kg body weight/day) are safe (Rana et al., 2006). Moreover, organophosphate compounds such as allicin have been reported to produce toxicity on the autonomic nervous system by suppressing cholinesterase; thereby, serving as an indirect-acting muscarinic agonist (Alare and Alare, 2020).
CONCLUSION
The results obtained in our work showed that GLf waste from garlic harvest processing could be used as an easily accessible source of natural bioactive compounds. The GLf extracted by water and 70% ethanol demonstrated a good quality standard of physical and chemical properties. Moreover, GLf extracts are rich in total phenolic and flavonoid compounds as well as antioxidant and antibacterial activities. Phenolic compounds including gallic acid, catechin and tannic acid were detected in the aqueous and ethanolic extracts of GLf. Flavonoid compounds including isoquercetin, quercetin, kaempferol and rutin were detected in the aqueous extract, whereas apigenin, isoquercetin, quercetin and kaempferol were found in the ethanolic of GLf. The ethanolic extract of GLf demonstrated the stronger antioxidant activity than aqueous extract using DPPH assay. The antioxidant activity of both aqueous and ethanolic extracts might associate with these phytochemical compounds. Moreover, GLf extracts exhibited antimicrobial activity against pathogenic bacteria with MIC and MBC values of 125-250 mg/mL. In addition, GLf aqueous extract showed lower toxicity on the Vero cells than ethanolic extract. Therefore, GLf waste comprises a promising source of natural antioxidants that can be used for various biological, medicinal, and food applications.
ACKNOWLEDGMENTS
The authors would like to express their sincere gratitude to the Faculty of Pharmacy, Chiang Mai University, Thailand for their financial support. The Office of the Permanent Secretary, Ministry of Higher Education, Science, Research, and Innovation (OPS-MHESI), Thailand and the Industrial Promotion Center Region 1, Ministry of Industry, Thailand were also acknowledged. The authors would like to thank the Division of Microbiology, Department of Biology, Faculty of Science, Chiang Mai University. We also would like to express our thanks to Thomas McManamon for language editing.
AUTHOR CONTRIBUTIONS
All authors contributed to the review and are responsible for the article content.
CONFLICT OF INTEREST
The authors declare they have no conflicts of interest. The authors alone are responsible for the content and writing of this article.
REFERENCES
Abiy, E., and Berhe, A. 2016. Anti-bacterial effect of garlic (Allium sativum) against clinical isolates of Staphylococcus aureus and Escherichia coli from patients attending Hawassa Referral hospital, Ethiopia. Journal of Infectious Diseases and Treatment. 2(2): 1-5.
Abubakar, E.M. 2009. Antibacterial activity of crude extracts of Euphorbia hirta against some bacteria associated with enteric infections. Journal of Medicinal Plants Research. 3: 498-505.
Alare, K., and Alare, T. 2020. Review of toxicity of allicin from garlic. Open Access Journal of Toxicology. 4(5): 00132- 00133.
Bayan, L., Koulivand, P.H., and Gorji, A. 2014. Garlic: a review of potential therapeutic effects. Avicenna Journal of Phytomedicine. 4(1): 1–14.
Beato, V.M., Orgaz, F., Mansilla, F., and Montaño, A. 2011. Changes in phenolic compounds in garlic (Allium sativum L.) owing to the cultivar and location of growth. Plant Foods for Human Nutrition. 66(3): 218-223.
Borges, A., Ferreira, C., and Saavedra, M.J. 2013. Antibacterial activity and mode of action of ferulic and gallic acids against pathogenic bacteria. Microbial Drug Resistance. 19: 256–265.
Chen, C.C., and Huang, C.Y. 2011. Inhibition of Klebsiella pneumoniae DnaB helicase by the flavonol galangin. The Protein Journal. 30: 59–65.
Chhouk, K., Uemori, C., Kanda, H., and Goto, M. 2017. Extraction of phenolic compounds and antioxidant activity from garlic husk using carbon dioxide expanded ethanol. Chemical Engineering and Processing: Process Intensification. 117: 113-119.
Clinical and Laboratory Standards Institute (CLSI). 2018. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically. 11th edition. Clinical and Laboratory Standards Institute. USA.
Cushnie, T.T., and Lamb, A.J. 2011. Recent advances in understanding the antibacterial properties of flavonoids. International Journal of Antimicrobial Agents. 38: 99–107.
Devi, A., Rakshit, K., Sarania, B., and Apatani, P. 2014. Ethnobotanical note on Allium species of Arunachal Pradesh, India. Indian Journal of Traditional Knowledge. 13(3): 606-612.
Djeridane, A., Yousfi, M., Nadjemi, B., Boutassouna, D., Stocker, P., and Vidal, N. 2006. Antioxidant activity of some Algerian medicinal plants extracts containing phenolic compounds. Food Chemistry. 97: 654-660.
Fukaya, M., Nakamura, S., Hayashida, H., Noguchi, D., Nakashima, S., Yoneda, T., and Matsuda, H. 2020. Structures of cyclic organosulfur compounds from garlic (Allium sativum L.) leaves. Frontiers in Chemistry. 8(282): 1-8.
Gunda, M.M., Prasad, R., Odelu, G., and Ugandhar, T. 2018. Studies on antimicrobial activity of garlic (Allium sativum L.) extract against Bacillus subtilis and Aspergillus niger. International Journal of Current Advanced Research. 7(1): 9169-9171.
Gupta, R., Gupta, M. K., Bhandari, A., and Gupta, J. 2014. Preliminary pharmacognostical and physicochemical analysis: A poly herbomineral formulation. International Journal of Drug Development and Research. 6(3): 85-92.
Harris, J.C., Cottrell, S., and Lloyd, D. 2001. Antimicrobial properties of Allium sativum (garlic). Applied Microbiology and Biotechnology. 57: 282–286.
Huang, Y.H., Huang, C.C., Chen, C.C., Yang, K. and Huang, C.Y. 2015. Inhibition of Staphylococcus aureus PriA helicase by flavonol kaempferol. The Protein Journal. 34: 169–172.
Jikihara, H., Qi, G., Nozoe, K., Hirokawa, M., Sato, H., and Sugihara Y. 2015. Aged garlic extract inhibits 1, 2-dimethylhydrazine-induced colon tumor development by suppressing cell proliferation. Oncology Reports. 33: 1131–1140.
Kallel, F., and Chaabouni, S.E. 2017. Perspective of garlic processing wastes as low-cost substrates for production of high-added value products: a review. Environmental Progress & Sustainable Energy. 36(6): 1-13.
Kallel, F., Driss, D., Chaari, F., Belghith, L., Bouaziz, F., Ghorbel, R., and Chaabouni, S.E. 2014. Garlic (Allium sativum L.) husk waste as a potential source of phenolic compounds: Influence of extracting solvents on its antimicrobial and antioxidant properties. Industrial Crops and Products. 62: 34-41.
Khelfi, S., Zerizer, S., Foughalia, A., Tebibel, S., Bensouici, C., and Kabouche, Z. 2023. The antioxidant activity and the anti-inflammatory effect of Citrus sinensis L. fruit on intestinal inflammation induced by hyperhomocysteinemia in mice. Natural and Life Sciences Communications. 22(1): e2023009.
Kumar, S., and Pandey, A.K. 2013. Chemistry and biological activities of flavonoids: an overview. Scientific World Journal. 162750: 1-16.
Likittrakulwong, W., Chanburee, S., Kitpot, T., Ninjiaranai, P., and Pongpamorn, P. 2023. Phytochemical properties, in vitro antimicrobial, and bioactive compounds of banana peel extractions using GC-MS. Natural and Life Sciences Communications. 22(2): e2023021.
Liu, Q., Wu, F., Chen, Y., Alrashood, S.T., and Alharbi, S.A. 2021. Anti-human colon cancer properties of a novel chemotherapeutic supplement formulated by gold nanoparticles containing Allium sativum L. leaf aqueous extract and investigation of its cytotoxicity and antioxidant activities. Arabian Journal of Chemistry. 14(4): 103039.
Londhe, V.P., Gavasane, A.T., Nipate, S.S., Bandawane, D.D., and Chaudhari P.D. 2011. Role of garlic (Allium sativum) in various diseases: An overview. Journal of Pharmaceutical Research and Opinion. 1(4): 129-134.
Majewski, M. 2014. Allium sativum: facts and myths regarding human health. Annals of the National Institute of Hygiene. 65: 1–8.
Mandal, M., Misra, D., Ghosh, N.N., and Mandal, V. 2017. Physicochemical and elemental studies of Hydrocotyle javanica Thunb. for standardization as herbal drug. Asian Pacific Journal of Tropical Biomedicine. 7(11): 979-986.
Maufaey, W., Sakkatat, P., Fongmul, S., and Kruekum, P. 2022. The model development of garlic production and marketing management of farmers in Chiang Mai. Journal of Agricultural Research and Extension. 39(3): 123-134.
Meriga, B., Mopuri, R., and Murali-Krishna, T. 2012. Insecticidal, antimicrobial and antioxidant activities of bulb extracts of Allium sativum. Asian Pacific Journal of Tropical Medicine. 5: 391–395.
Mishra, A., Mishra, A.K., Ghosh, A.K., and Jha, S. 2011. Pharmacognostical, physicochemical and phytochemical studies of some marketed samples of roots used in ayurvedic medicines. Pharmacognosy Journal. 3(24): 55-61.
Mohsen, A., and Shahab, B., 2010. Introducing of green garlic plant as a new source of allicin. Food Chemistry. 120: 179–183.
Morihara, N., Hino, A., Miki, S., Takashima, M., and Suzuki, J.I. 2017. Aged garlic extract suppresses inflammation in apolipoprotein E-knockout mice. Molecular Nutrition and Food Research. 61: 1-7.
Naji, K.M., Al-Shaibani, E.S., Alhadi, F.A, and D’souza, M.R. 2017. Hepatoprotective and antioxidant effects of single clove garlic against CCl 4-induced hepatic damage in rabbits. BMC Complementary and Alternative Medicine. 17(411): 1-12.
Osbole, O.O., Gbolade, A.A., and Ajaiyeoba, E.O. 2010. Ethnobotanical survey of plants used in treatment of inflammatory diseases in Ogun state of Nigeria. European Journal of Scientific Research. 43(2): 183-191.
Panche, A.N., Diwan, A.D., and Chandra, S.R. 2016. Flavonoids: an overview. Journal of Nutritional Science. 5: 1-15.
Penarrieta, J.M., Alvarado, J.A., Akesson, B., and Bergenstahl, B. 2007. Separation of phenolic compounds from foods by reversed-phase high performance liquid chromatography. Revista Boliviana de Química. 24(1): 1-4.
Petkova, N., Bileva, T., Valcheva, E., Dobrevska, G., Grozeva, N., Todorova, M., and Popov, V. 2023. Non-polar fraction constituents, phenolic acids, flavonoids and antioxidant activity in fruits from florina apple variety grown under different agriculture management. Natural and Life Sciences Communications. 22(1): e2023012.
Qiu, Z., Qiao, Y., Zhang, B., Sun-Waterhouse, D., and Zheng, Z. 2022. Bioactive polysaccharides and oligosaccharides from garlic (Allium sativum L.): Production, physicochemical and biological properties, and structure–function relationships. Comprehensive Reviews in Food Science and Food Safety. 21: 3033–3095.
Rader, H., and McGuinnes, J. 2022. Growing Garlic in Alaska. University of Alaska Fairbanks. Alaska, USA. pp. 1-8.
Rana, S.V., Pal, R., Vaiphei, K., and Singh, K. 2006. Garlic hepatotoxicity: Safe dose of garlic. Tropical Gastroenterology. 27(1): 26-30.
Sharma, N. 2019. Efficacy of garlic and onion against virus. International Journal of Research in Pharmaceutical Sciences. 10: 3578–3586.
Sharma, P., Mohanty, J. P., Ghosh, P., Sharma, C., and Subba, B. 2019. Pharmacognostical and preliminary phytochemical investigation of Equisetum debile Roxb. Journal of Drug Delivery and Therapeutics. 9(3-s): 163-169.
Singh, R., and Singh, K. 2019. Garlic: a spice with wide medicinal actions. Journal of Pharmacognosy and Phytochemistry. 8(1): 1349–1355.
Soladoye, M.O., Adetayo, M.O., Chukwuma, E.C., and Adetunji, A.N. 2010. Ethnobotanical survey of plants used in the treatment of haemorrhoids in South-Western Nigeria. Annals of Biological Research. 1(4): 1-15.
Stratil, P., Klejdus, B., and Kubáň, V. 2006. Determination of total content of phenolic compounds and their antioxidant activity in vegetables evaluation of spectrophotometric methods. Journal of Agricultural and Food Chemistry. 54(3): 607–616.
Subroto, E., Cahyana, Y., Tensiska, M., Lembong, F., Filianty, E., and Kurniati E. 2021. Bioactive compounds in garlic (Allium sativum L.) as a source of antioxidants and its potential to improve the immune system: A review. Food Research. 5: 1-11.
Takashima, M., Kanamori, Y., Kodera, Y., Morihara, N., and Tamura, K. 2017. Aged garlic extract exerts endothelium-dependent vasorelaxant effect on rat aorta by increasing nitric oxide production. Phytomedicine. 24: 56–61.
Tesfaye, A., and Mengesha, W. 2015. Traditional uses, phytochemistry and pharmacological properties of garlic (Allium Sativum) and its biological active compounds. International Journal of Engineering Research and Technology. 1: 142–148.
Thai Herbal Pharmacopoeia (THP). 2022. Thai Herbal Pharmacopoeia 2021. Nonthaburi: Department of Medical Sciences, Ministry of Public Health.
Thomson, M., and Ali, M. 2003. Garlic [Allium sativum]: A review of its potential use as an anti-cancer agent. Current Cancer Drug Targets. 3: 67–81.
Tudu, C.K., Dutta, T., Ghorai, M., Biswas, P., Samanta, D., Oleksak, P., Jha, N.K., Kumar, M., Radha, Procków, J., ´Pérez de la Lastra, J.M., and Dey, A. 2022. Traditional uses, phytochemistry, pharmacology and toxicology of garlic (Allium sativum), a storehouse of diverse phytochemicals: A review of research from the last decade focusing on health and nutritional implications. Frontiers in Nutrition. 9: 1-19.
Umthong, S., Phuwapraisirisan, P., Puthong, S., and Chanchao, C. 2011. In vitro antiproliferative activity of partially purified Trigona laeviceps propolis from Thailand on human cancer cell lines. BMC Complementary and Alternative Medicine. 11(37): 1-8.
Warinthip, N., Liawruangrath, B., Natakankitkul, S., Rannurags, N., Pyne, S.G. and Liawruangrath, S. 2023. Chemical constituents antioxidant and antibacterial activities of the leaves and flowers from Gardenia carinata wallich. Natural and Life Sciences Communications. 22(1): e2023003.
Wojdylo, A., Oszmianski, J., and Czemerys, R. 2007. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chemistry. 105: 940-949.
OPEN access freely available online
Natural and Life Sciences Communications
Chiang Mai University, Thailand. https://cmuj.cmu.ac.th
Panee Sirisa-ard1, Kiatisak Pholsonklam2, Apinya Satchachai3, Yingmanee Tragoolpua4, 5 and Thida Kaewkod4, 5, *
1 Faculty of Pharmacy, Chiang Mai University, Chiang Mai 50200, Thailand.
2 Faculty of Science, Payap University, Chiang Mai 50200, Thailand.
3 Northern Siam Seedlac Co., Ltd., Lampang 52100, Thailand.
4 Division of Microbiology, Department of Biology, Faculty of Science, Chiang Mai University, Chiang Mai 50200, Thailand.
5 Natural Extracts and Innovative Products for Alternative Healthcare Research Group, Faculty of Science, Chiang Mai University, Chiang Mai 50200, Thailand.
Corresponding author: Thida Kaewkod E-mail: thida.kaewkod@cmu.ac.th
Total Article Views
Editor: Sirasit Srinuanpan,
Chiang Mai University, Thailand
Article history:
Received: April 22, 2023;
Revised: June 18, 2023;
Accepted: July 21, 2023;
Online Frist: August 9, 2023

