
Antioxidant, Anti-Tyrosinase, Antiglycation and Safety of Longan Leaf Extract for Cosmeceutical Application
Pimjai Doungsaard, Sunee Chansakaow, Worrapan Poomanee, Jakkapan Sirithunyalug, Nutjeera Intasai, and Pimporn Leelapornpisid*Published Date : July 10, 2023
DOI : https://doi.org/10.12982/NLSC.2023.052
Journal Issues : Number 3, July-September 2023
Abstract Many potential bioactive phytoconstituents in longan (Dimocarpus longan Lour.) leaf have been reported. Most chemical compounds belong to either phenolics or flavonoids, known as potent antioxidants. This research explores the multifunctional potential as a bioactive cosmetic ingredient used in skin care for adding value to longan leaves. The dried powder of longan leaves was extracted with 50%(v/v) ethanol in de-ionized water, then quantified for total phenolic and flavonoid contents. The hydroethanolic extract was investigated for various antioxidant methods including DPPH, nitric oxide (NO) scavenging and lipid peroxidation inhibition assays. The anti-tyrosinase activity was also evaluated for skin-whitening properties. Furthermore, Anti-aging activity in term of antiglycation was also conducted. The high number of polyphenols within the extract possessed a high antioxidant potential with IC50 0.023 ± 0.002, 0.478 ± 0.033 and 1.495 ± 0.153 mg/ml for DPPH scavenging, NO scavenging and lipid peroxidation inhibition, respectively. The extract could inhibit the tyrosinase enzyme with IC50 4.90 ± 0.50 and 6.60 ± 0.18 mg/ml for L-tyrosine and L-DOPA as substrates. The extract also exhibited a potent antiglycation activity with IC50 0.023 ± 0.003 mg/ml. Moreover, it showed no irritation effect on the chorioallantoic membranes (CAMs) within 5 min using the Hen’s Egg Test-Chorioallantoic Membranes (HET-CAMs). In conclusion, the polyphenolic-rich longan leaf extract displayed a good antioxidant, anti-tyrosinase and excellent antiglycation potential with safety that could constitute a promising multifunctional bioactive ingredient for skin care cosmeceutical products.
Keywords: Longan leaf extract, Antioxidants, Anti-tyrosinase, Antiglycation, Irritation, HET-CAMs, Cosmeceutical
Funding: The authors are grateful for the research funding provided by the Fundamental Fund 2022 from National Research Council of Thailand for providing financial support to conduct this study.
Citation: Doungsaard, P., Chansakaow, S., Poomanee, W., Sirithunyalug, J., Intasai, N., and Leelapornpisid, P. 2023 Antioxidant, anti-tyrosinase, antiglycation and safety of longan leaf extract for cosmeceutical application. Natural and Life Sciences Communications. 22(3): e2023052.
INTRODUCTION
Polyphenols are plant metabolites in which one or more phenolic hydroxyl groups are bound to benzene ring systems in the chemical structure. Multiple studies have reported that polyphenol-enriched plant extracts could effectively prevent and treat dermatologic disorders. The beneficial properties for cosmetic applications include antioxidant activity, skin UV damage protection, dermal enzymatic reaction inhibition and antimicrobial effect. (Wittenauer et al., 2015; Zillich et al., 2015; Schlay et al., 2016). Due to the profitability of polyphenols, they have recently attracted attention in the cosmetic industry.
Longan (Dimocarpus longan Lour.) is a well-known tree species of the Sapindaceae family, widely cultivated in Chiang Mai, Lamphun and Chiang Rai for commercial purposes as the largest proportion in Northern Thailand (Chandarasrivong, R., 2019). Longan leaves constitute an agricultural waste requiring appropriate handling under environmentally friendly methods. Longan leaf extracts were reported to possess numerous bioactive contents especially phenolic and flavonoid compounds (Liu et al., 2012; Xue et al., 2015). Various research investigations have revealed the in vitro biological activities of the extracts. In a study of Ripa et al. (2010), methanolic longan leaf extract was semi-purified using liquid-liquid extraction with various solvents. The extracts showed the ability to scavenge DPPH radicals at the same level as ascorbic acid. Chen et al. (2017) reported that the 40% ethanol extract from 1 g of dried longan leaf powder exhibited DPPH and ABTS radicals scavenging ability equivalent to Trolox at 218.67 and 582.37 µmol/g, respectively. In addition, the extract showed ferric-reducing antioxidant power (FRAP) equivalent to 182.58 µmol of Trolox. Ripa et al. (2010) also reported that the leaf extracts could exhibit anti-bacterial activity with 16 species of bacteria. In the study of Apriyanto et al. (2016), methanol leaf extract could inhibit the activity of the hepatitis-C virus genotype 2a strain JFH1. Doungsaard et al. (2020) declared the anti-aging potential of hydroethanolic extract in that it showed the hyaluronidase and collagenase inhibiting activities with IC50 of 234.80 ± 21.52 and 314.44 ± 62.14 µg/mL, respectively.
This research aimed to determine the phenolic contents and investigate more varied biological activities, including antioxidant, anti-tyrosinase, antiglycation and ex vivo irritation effect of hydroethanolic longan leaf extract to confirm its multifunctional properties and safety for rinse-off and leave-on cosmeceutical products.
MATERIALS AND METHODS
Chemical and reagent
Deionized water was generated by Millipore Milli-Q Advantage procured from Merck. (Darmstadt, Germany). Bovine serum albumin, Folin-Ciocalteu’s reagent, 2,2’-azobis (2- amidinopropane) dihydrochloride (AAPH), 2,2-diphenyl-1-picrylhydrazyl (DPPH) and phosphoric acid were obtained from Merck (Darmstadt, Germany). Trolox, aluminum chloride (AlCl3), ammonium thiocyanate (NH4SCN), calcium chloride (CaCl2), gallic acid, iron (II) chloride tetrahydrate (FeCl2•4H2O), L-tyrosine, L-3,4-dihydroxyphenylalanine (L-DOPA), linoleic acid, phosphoric acid (H3PO4), N-acetyl-l-carnosine, naphthylethylenediamine dihydrochloride, sodium hydroxide (NaOH), sodium nitrite (NaNO2), sodium nitroprusside, sulfanilamide, Tween 20® and tyrosinase mushroom enzyme were purchased from Sigma-Aldrich (Steinheim, Germany). Additionally, 95% ethanol, methanol, potassium persulfate (K2S2O8) and sodium carbonate (Na2CO3) were purchased from RCI Labscan (Bangkok, Thailand). All the chemicals were analytical grade.
Plant materials and preparation of crude polyphenol extract
Longan leaf was harvested from January to February 2021 from a longan farm in Lamphun Province, Thailand. Mature leaves were washed and dried using a hot air oven at 50ºC. Then they were ground to a fine powder and macerated with 50% ethanol three times for 48 hours at room temperature. The solution extracts were pooled, filtered, and evaporated to dryness under 50 mbar on a rotary evaporator (BUCHI, R-300, Switzerland) at 50 °C. The crude extract was then stored at -20 °C for further experiments.
Total phenolic content determination
The Folin-Ciocalteau method of Theansungnoen et al. (2022) was performed to quantify the phenolic content. After incubating the mixture, composed of 0.5 ml of the sample, 2 ml of 10% (v/v) Folin-Ciocalteau reagent and 4 ml of 7.5% (w/v) Na2CO3, the reaction solution absorbance was measured at 765 nm using a UV-Vis spectrophotometer (UV-2600i, Shimadzu, Kyoto, Japan). The equivalent concentration to the standard calibration curve of gallic acid was calculated following the linear equation y = 0.0076x + 0.0141 where y is the absorbance of reacted gallic acid and x is the concentration; correlation coefficient of R2 = 0.9998, was offered to express the total phenolic amount. The result was presented as mg of gallic acid equivalents (mg GAE/g of the dried extract).
Total flavonoid content determination
The aluminum chloride colorimetric method of Theansungnoen et al. (2022) was performed to quantify total flavonoids. The reaction mixture containing 1 ml of sample, 7.4 ml of deionized (DI) water, 0.3 ml of 5% (w/v) NaNO2 and 0.3 ml of 10% (w/v) AlCl3 was incubated for 6 min. After adding 2 ml of 1 M NaOH, the absorbance was measured at 510 nm using a UV-Vis spectrophotometer (UV-2600i, Shimadzu, Kyoto, Japan. The total flavonoid content was offered at an equivalent concentration to the standard calibration curve of quercetin, which was calculated following the linear equation y = 0.0011x + 0.0052 where y is the absorbance of reacted quercetin and x is the concentration; the correlation coefficient of R2 = 0.9998. The result was presented as mg of quercetin equivalents (mg QE/g of the dried extract).
Antioxidant activity
DPPH radical scavenging assay
DPPH scavenging activity assay was performed following the method of Kamma et al. (2019). The sample was diluted with 50% (v/v) ethanol from 0.01 to 1 mg/ml. Trolox and gallic acid, serving as standards, were diluted in the same solvent at seven serial concentrations. The reaction was started by mixing 20 µl of the extract solution with 180 µl of DPPH ethanolic solution in a 96-well plate. After the 30 min incubation at room temperature, the solution was measured for absorbance at 520 nm using a microplate reader (SPECTROstar Nano®, Ortenberg, Germany). The DPPH radicals scavenging effect was represented as 50% inhibitory concentration (IC50 value) and a percentage of DPPH decolorization using the equation below.
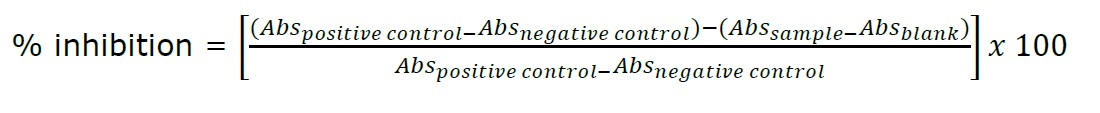
where Abspositive control is the absorbance value of the mixture containing solvent and DPPH solution, Absnegative control is the absorbance value of the positive control when DPPH solution is replaced with absolute ethanol, Abssample is the absorbance value of reaction mixture containing the sample and DPPH solution and Absblank is the absorbance value of the reaction mixture when the DPPH solution is replaced with absolute ethanol.
Nitric oxide scavenging assay
The method of Jagetia and Baliga (2004) was performed to determine the NO scavenging activity. The extract was diluted with 50% (v/v) ethanol to 0.60 to 20 mg/ml. Gallic acid, serving as a standard, was diluted in the same solvent at seven serial concentrations. After mixing 80 µl of sodium nitroprusside (10 mM) in phosphate buffer saline (PBS, pH 7.4) with 20 µl of extract, the mixture was incubated at 25 °C for 150 min. Finally, 100 µl of Griess reagent (1% sulfanilamide, 2.5% H3PO4 and 0.1% naphthylethylenediamine dihydrochloride) was added for nitrite detection. The solution was measured for absorbance at 540 nm using a microplate reader (SPECTROstar Nano®, Ortenberg, Germany). The ability to scavenge NO of samples was calculated and expressed as 50% inhibitory concentration (IC50 value) and a percentage of inhibition using the equation below.

where Abspositive control and Absnegative control represent the absorbance of the mixture containing the 50% (v/v) ethanol, Griess reagent with and without sodium nitroprusside; Abssample and Absblank represent the absorbance of the reaction mixture containing the sample, and Griess reagent, with and without sodium nitroprusside.
Linoleic peroxidation inhibition
Linoleic peroxidation inhibition was investigated by ferric thiocyanate (FTC) assay using the method of Theansungnoen et al. (2022). The extract was diluted with 50%(v/v) ethanol to make a concentration ranging from 0.60 to 20 mg/ml. Trolox and gallic acid, serving as standard, were diluted in the same solvent at seven serial concentrations. The reaction mixture was prepared by mixing 175 µl of DI water, 350 µl of 20 mM phosphate buffer pH 7.0, 350 µl of 1.3% (v/v) linoleic acid in methanol and 150 µl of the sample. Then 50 µl of AAPH was added and incubated at 45 °C for 4 h. After incubating, 50 µl of the reaction mixture was added to 50 µl of 20 mM iron (II) chloride solution, 50 µl of 10% (w/v) ammonium thiocyanate solution and 4.85 ml of 75% (v/v) methanol for 3 min. Then the mixture was measured at 500 using a UV-Vis spectrophotometer (UV-2600i, Shimadzu, Kyoto, Japan). The absorbance of control was determined by replacing the sample with 50% (v/v) ethanol. The results were reported as IC50 value and a percentage of linoleic peroxidation inhibition using the equation below.

where Abssample represents the absorbance of the reaction mixture containing all reagents mentioned above, and Abscontrol represents the absorbance of the reaction mixture, replacing the sample with 50% (v/v) ethanol.
Anti-tyrosinase assay
Anti-tyrosinase was determined using the dopachrome method of Kamma et al. (2019). The extract was diluted in 20%(w/w) Tween 20® to 1.50 to 50 mg/ml. Gallic acid and kojic acid, used as standard, were diluted in the same solvent at seven serial concentrations. The reaction mixture was started by mixing 70 µl of extract solution with 70 µl of 100 units of mushroom tyrosinase in 20 mM phosphate buffer pH 6.8. After incubating at 37 °C for 10 min, 70 µl of 0.85 mM L-tyrosine or L-DOPA in 20 mM phosphate buffer pH 6.8 was added. The absorbance was measured at 450 nm using a SPECTROstar nano (BMG LABTECH GmbH, Germany). The ability to inhibit the tyrosinase activity of samples was calculated and represented as 50% inhibitory concentration (IC50 value) and a percentage of inhibition using the equation below.

where Abspositive control and Absnegative control represent the absorbance of the mixture containing the 20% (w/w) Tween 20®, substrate solution with and without mushroom tyrosinase; Abssample and Absblank represent the absorbance of the reaction mixture containing sample, substrate solution with and without tyrosinase.
Antiglycation assay
Antiglycation activity was evaluated followed the method of Povichit et al. (2010). The extract was diluted with 50%(v/v) ethanol to 0.30 to 10 mg/ml. Gallic acid and N-acetyl carnosine, serving as standards, were diluted in the same solvent at seven serial concentrations. The reaction started after mixing 300 µl of bovine serum albumin (20 mg/ml) with 300 µl of 0.5 M D-glucose in 200 mM potassium phosphate buffer saline (PBS, pH 7.4) and 100 µl of the sample. The reaction mixture was incubated at 45 °C for seven days. Fluorescence intensity was measured using a multimode microplate reader (Spectramax M3®, San Jose, CA, USA) at an excitation of 370 nm and emission of 440 nm. The fluorescence intensity of the control was determined by replacing the sample with 50% (v/v) ethanol. The antiglycation ability of all samples was expressed as a percentage of inhibition and calculated using the equation below. Then the IC50 was also calculated.

where F.I.sample represents the fluorescence intensity of the reaction mixture containing all reagents mentioned above and F.I.control represents the fluorescence intensity of the reaction mixture, which replaced the sample with 50% (v/v) ethanol.
Irritation determination using hen’s egg test-chorioallantoic membranes (HET-CAMs)
Longan leaf extract was investigated for irritation using the HET-CAMs assay following Yeerong et al. (2021). The extract was dissolved in 20% (w/w) Tween 20® at 5 mg/ml concentration. The fertilized hen’s eggs were incubated in an incubator at 37 ± 1 °C and 55 ± 0.5% relative humidity for 7 days. The incubated eggs are opened carefully, exposing the chorioallantoic membrane (CAMs). After that, 30 µl of the extract solution was dropped directly on the CAMs. The reaction outcome, including vascular hemorrhage, lysis and coagulation, was observed via a stereo microscope (Olympus, Tokyo, Japan). The observation time was 5 min period. An irritation score (IS) is calculated by the following equation.
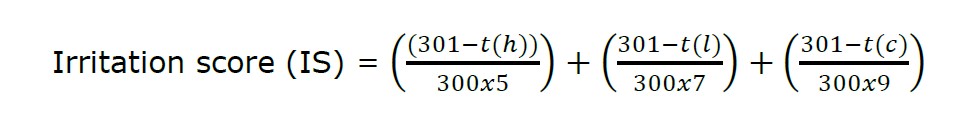
where t(h) is the time (sec) when the first sign of vascular hemorrhages arises, t(l) is the time (sec) when first the sign of vascular lysis arises, and t(c) is the time (sec) when the sign of vascular coagulation first arises.
Statistical analysis
Three replications from all experiments expressed data as mean ± standard deviation (SD). ANOVA and Tukey's post hoc test using SPSS Program Software, Version 25, was used to analyze statistical analysis. The value of P <0.05 defined a statistical significance.
RESULTS AND DISCUSSION
Longan leaf extract
This research was a continuation of our related study, comparing some anti-aging activity of the ethanolic and hydroethanolic longan leaf extracts. The study revealed that the hydroethanolic extract produced a higher yield with remarkably superior in vitro biological activity compared with ethanolic extract (Doungsaard et al., 2020). According to the study of Thoo et al. (2010), ethanol concentration affected polyphenol content. The yield of total phenolic content dropped considerably with further increases in more than 60% ethanol, while the yield of total flavonoid content declined with further increases in ethanol. However, the extraction with a monosolvent system (100% ethanol) might not contribute to high antioxidant capacities.
In this study, the selected hydroethanolic extract using the same extracting procedure but a different cultivated area and time of longan leaves, was repeated to confirm the reproducibility of the extraction. The longan leaves which were macerated using 50%(v/v) ethanol yielded 20.98 ± 1.29 % (w/w) of a sticky crude extract presenting a brown appearance. For total phenolic and flavonoid contents, the leaf extract contained a high content of both phenolic and flavonoid compounds at 363.61 ± 21.74 mg GAE/g of dry weight extract and 432.77 ± 27.31 mg QE/g of dry weight extract, respectively. Compared with a related study (Doungsaard et al., 2020), total phenolic content was similar (373.67 ± 13.25 mg GAE/g of dry weight extract). However, the total flavonoid content was higher than our study with a value of 262.75 ± 18.34 mg QE/g of dry-weight extract. The different amounts of total phenolic and flavonoid contents from a related study could have been due to different factors including cultivated area, seasonal changes and harvesting time (Li et al., 2008). The quality control of the extract should be further adopted for reproducibility in cosmeceutical applications.
Antioxidant properties
Antioxidant activities of longan leaf extract were determined by DPPH scavenging, NO scavenging and inhibiting lipid peroxidation because multiple reactions and mechanisms are related in the antioxidant process. Reactive oxygen species (ROS) are the initiators of the aging process. DPPH scavenging assays showed the ability of tested compounds to eliminate free radicals. Additionally, the cell membranes' polyunsaturated fatty acid could induce lipid peroxidation by ROS. Lipid organization is important for skin barrier functions, so inhibiting lipid peroxidation is the most biologically important method to apply in cosmetics and cosmeceuticals (Theansungnoen et al., 2022; Bouwstra and Ponec, 2006). NO is naturally generated in the skin as a neural transistor for an immune response. However, the elevation of NO level may result in DNA or protein damage by reacting with oxygen to produce highly genotoxic intermediates (Jagetia and Baliga, 2004).
Because the ability of longan leaf extracts as NO scavengers has not been reported before, therefore, the NO scavenging ability of the extract needed to be determined. The hydroethanolic longan leaf extract showed good NO scavenging activity. The high amount of polyphenol extract possessed a high antioxidant potential. The IC50 values of each assay are shown in Table 1. According to a related study (Doungsaard et al., 2020), 95% (v/v) ethanol and 50% (v/v) ethanol extract presented a different ratio in total phenolic and flavonoid contents. The bioactivities of the extracts might be related to other groups of phenolic compounds in addition to flavonoids. DPPH scavenging assay and linoleic acid peroxidation inhibition of longan leaf extract was repeated to confirm the ability of the extract compared with the related study. In this study, the extract expressed IC50 of DPPH scavenging activity at about 0.023 ± 0.002 mg/ml, a slightly better result and agreeing with the related study with IC50 at about 0.030 ± 0.008 mg/ml. While the ability to inhibit linoleic acid peroxidation was decreased from the related study (IC50 of 0.537 ± 0.042 mg/ml) to IC50 of 1.495 ± 0.153 mg/ml, the ratio of phenolic to flavonoid content increased from 0.70 to 1.19 (Doungsaard et al., 2020). Thus, phenolic compounds, not only flavonoids, are believed to be attributed to antioxidant capacities, especially for inhibiting lipid peroxidation.
Table 1. IC50 of biological activities of longan leaf extract.
|
Sample |
IC50 (mg/ml) |
|||||
|
DPPH scavenging assay |
Nitric oxide scavenging assay |
Lipid peroxidation inhibitory assay |
Tyrosinase inhibition assay |
Antiglycation assay |
||
|
L-tyrosine substrate |
L-DOPA substrate |
|||||
|
Longan leaf extract |
0.023 ± 0.002a |
0.478 ± 0.033a |
1.495 ± 0.153a |
4.900 ± 0.500a |
6.600 ± 0.180a |
0.023 ± 0.003a |
|
Standards |
|
|
|
|
|
|
|
Trolox |
0.008 ± 0.003b |
- |
0.213 ± 0.010b |
- |
- |
- |
|
Gallic acid |
0.001 ± 0.002c |
0.050 ± 0.008b |
0.392 ± 0.019c |
0.120 ± 0.030b |
0.240 ± 0.130b |
- |
|
Kojic acid |
- |
- |
- |
0.014 ± 0.001c |
0.023 ± 0.001c |
- |
|
N-acetyl carnosine |
- |
- |
- |
- |
- |
10.740 ± 1.990b |
Note: - = not applicable, Each value represented the mean ± SD (n=3). In each column, different superscripts represent significant differences (P <0.05).
Anti-tyrosinase properties
Tyrosinase, a copper-containing enzyme, is principally involved in melanin synthesis and is believed to be the target of skin-whitening active ingredients by inhibiting its activity. The melanogenesis process is initiated by the oxidation of L-tyrosine to L-DOPA and dopaquinone by tyrosinase. Then the dopaquinone will serve as a substrate for further synthesizing melanin, called eumelanin and pheomelanin. L-DOPA was also the substrate for oxidizing to dopaquinone by the enzyme (Pillaiyar et al., 2017). The IC50 values of the longan leaf extract against tyrosinase were 4.900 ± 0.500 and 6.600 ± 0.180 mg/ml for L-tyrosine and L-DOPA substrates, respectively. The longan leaf extract indicated relatively lower inhibitory activity compared with positive controls, gallic acid (IC50: 0.120 ± 0.030 and 0.240 ± 0.130 mg/ml, for L-tyrosine and L-DOPA as substrates) and kojic acid (IC50: 0.014 ± 0.001 and 0.023 ± 0.001 mg/ml, for L-tyrosine and L-DOPA as substrates). However, the inhibitory effect on tyrosinase activity was dose-dependent, which could consider longan leaf extract as a skin-whitening agent. According to the total phenolic and flavonoid contents of the longan leaf extract, the anti-activity could depend on these compounds, as they were reported to obstruct the tyrosinase activity by chelating copper atoms (Xue et al., 2011). The hydroethanolic longan leaf extract contained gallic acid and ellagic acid, reporting a strong tyrosinase activity (Kim, 2007; Huang et al., 2019). These compounds were involved in inhibiting tyrosinase activity but might be interrupted by other compounds in the extract. Therefore, further purification should be employed for skin-whitening application.
Antiglycation
Glycation is a naturally aging reaction involving both extrinsic and intrinsic factors. The reactions start as bridging reactions between hydroxyl groups of reducing sugars and free amino groups from dermal proteins. Then, the dicarbonyl compounds propagate, producing advanced glycation products (AGEs). Excessive AGEs have been confirmed to cause skin aging or so-called age spots. Polyphenols have been confirmed to have antiglycation functions in vivo and in vitro. Phenolic acids and flavonoids can inhibit glucose-mediated protein modification, which might be associated with antioxidant function. Moreover, the hydroxyl groups of polyphenols have excellent dicarbonyl compound trapping functions. In addition, the use of two or more polyphenol substances could exhibit better antiglycation ability than using single substance, which could explain the very strong antiglycation potential of longan leaf extract (IC50: 0.023 ± 0.003 mg/ml) that is superior to n-acetyl carnosine (IC50: 10.740 ± 1.990 mg/ml), the commercial dipeptide for antiglycation (Reddy et al., 2005; Yeh et al., 2017).
Irritation property by HET-CAMs
The HET-CAMs assay is a test used to determine cosmetic products' irritation properties. This assay is one of the oldest and most alternatively used to the Draize test. The available protocols are the visualization of irritant reaction within 300s as mimicking the tear film turnover in humans. However, the products can be in contact with the CAMs constantly, relying on the purpose of cosmeceutical applications (Rivero et al., 2021). For long term irritation, the observation was continuously observed until 60 min. Table 2 presents the IS values of longan leaf extract, control and solvent used in the HET-CAMs assay from 5 min observation. IS 0.0 to 0.9, is classified as nonirritation, 1.0 to 4.9 as slight irritation, 5.0 to 8.9 as moderate irritation and 9.0 to 21.0 as severe irritation. DI water with 1% (w/v) SLS was used as a positive control because it reportedly causes acute skin irritation (Yeerong et al., 2021). In this study, SLS also showed severe irritation, including hemorrhage, coagulation, and vascular lysis, with an IS score of 10.0 ± 0.5.
Table 2. Irritation score and irritation assessment from the HET-CAM assay (n=3).
|
Sample |
Irritation score |
Irritation level |
|
Positive control (1% w/v SLS) |
12.5 ± 0.3 |
Severe irritation |
|
Negative control (0.9% w/v NaCl) |
0.0 ± 0.0 |
No irritation |
|
Vehicle (DI water) |
0.0 ± 0.0 |
No irritation |
|
Vehicle (20% w/w Tween 20®) |
0.0 ± 0.0 |
No irritation |
|
Longan leaf extract (5 mg/ml in 20% w/w Tween 20® in DI water |
0.0 ± 0.0 |
No irritation |
In contrast, the negative control or 0.9% (w/v) NaCl in DI water presented non-irritation. DI water, and 20% Tween 20® in DI water was investigated as vehicle controls, presenting no sign of irritation in 5 min, and interpreted as no irritation with an IS score of 0.0 ± 0.0. The solution of the extract (5 mg/ml) in 20% (w/w) Tween 20® in DI water also presented no sign of irritation in 5 min and was interpreted as no irritation with an IS score of 0.0 ± 0.0. The result could indicate that longan leaf extract was considered safe for topical use on human skin. The photographs of the CAMs and blood vessels before and after exposure to samples after 5 and 60 min are presented in Figure 1. The irritation effect of SLS was observed within 5 min after application, while 0.9% (w/v) NaCl and DI water were nonirritant in both short (5 min) and long (60 min) term observation. Tween 20® (20% w/w) showed non-irritant effects on CAM within 5 min but presented vascular coagulation after 60 min application. Longan leaf extract presented no irritation sign on CAMs within 5 min but found vascular hemorrhage and coagulation after 60 min application. This irritation effect might be from longan leaf extract or the vehicle. According to IS score, the longan leaf extract could be applied to rinse off and leave-on cosmetic products. However, after long term application, the extract or the vehicle might irritate the skin. Thus, in vivo methods for irritation assay should also be considered for long term application.
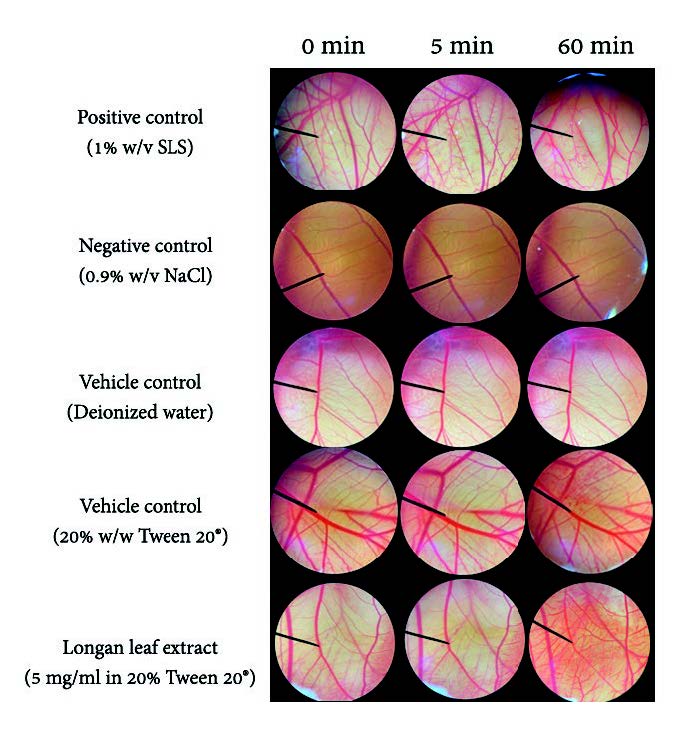
Figure 1. Effect of 1% (w/v) SLS (positive control), 0.9% (w/v) NaCl solution, DI water (vehicle control), 20% Tween 20® (vehicle control) and longan leaf extract on chorioallantoic membrane (0 min, after 5 min and 60 min exposure).
CONCLUSION
The polyphenol-rich longan leaf extract demonstrated a remarkable multifunctional biological activity including antioxidant, anti-tyrosinase and antiglycation, with in vitro studies. Furthermore, the extract showed nonirritation on the HET-CAM test. Therefore, polyphenol-rich longan leaf extract is a promising anti-aging, skin whitening natural ingredient and safe for rinse-off and leave-on skin care cosmetic applications.
ACKNOWLEDGMENTS
The authors would like to thank the Fundamental Fund 2022 from National Research Council of Thailand for providing financial support to conduct this study. Also, we would like to gratefully acknowledge the Faculty of Pharmacy, Chiang Mai University, Thailand, for the use of all facilities.
AUTHOR CONTRIBUTIONS
Pimporn Leelapornpisid designed, conducted all the experiments, performed the data visualization and wrote the manuscript. Pimjai Doungsaard assisted in conducting the experiments and performed the statistical analysis. All authors have read and approved of the final manuscript.
CONFLICT OF INTEREST
The authors declare that they hold no competing interests.
REFERENCES
Apriyanto, D.R., Aoki, C., Hartati, S., Hanafi, M. Kardono, L. B. S., Arsianti, A. Louisa, M., Sudiro, T.M., Dewi, B.E., Sudarmono, P. et al. 2016. Anti-hepatitis C virus activity of a crude extract from longan (Dimocarpus longan Lour.) leaves. Japanese Journal of Infectious Disease. 69(3): 213–220.
Bouwstra, J.A. and Ponec, M. 2006. The skin barrier is in a healthy and diseased state. Biochimica et Biophysica Acta (BBA)-Biomembranes. 1758(12): 2080-2095.
Chen, G.L., Zhang, X., Chen, S.G., Han, M.D., and Gao, Y.Q. 2017. Antioxidant activities and contents of free, esterified and insoluble-bound phenolics in 14 subtropical fruit leaves collected from the South of China. Journal of Functional Foods, 30: 290-302
Doungsaard, P., Chansakaow, S., Sirithunyalug, J., Shang-Chian, L., Wei-Chao, L. Chia-Hua, L. Kuan- Ha, L., and Leelapornpisid, L. 2020. In vitro biological activities of the anti-aging potential of Dimocarpus longan leaf extracts. Chiang Mai University Journal of Natural Science. 19(2): 235-51.
Jagetia, G.C., and Baliga, M.S. 2004. The evaluation of nitric oxide scavenging activity of certain Indian medicinal plants in vitro: A preliminary study. Journal of Medicinal Food. 7(3): 343-348.
Huang, Q., Chai, W. M., Ma, Z. Y., Deng, W. L., Wei, Q. M., Song, S., Zou, Z. R. and Peng, Y. Y. 2019. Antityrosinase mechanism of ellagic acid in vitro and its effect on mouse melanoma cells. Journal of Food Biochemistry, 43(11): e12996.
Kamma, M., Wei-Chao, L., Lau, S-C., Chansakaow, S., and Leelapornpisid, P. 2019. Anti-aging cosmeceutical product containing of Nymphaea rubra Roxb.ex Andrews extract. Chiang Mai Journal of Science. 46(6): 1143-1160.
Kim, Y. J. 2007. Antimelanogenic and antioxidant properties of gallic acid. Biological and Pharmaceutical Bulletin, 30(6), 1052–1055.
Li, S., Han, Q., Qiao, C., Song, J., Lung Cheng, C., and Xu, H. 2008. Chemical markers for the quality control of herbal medicines: an overview. Chinese Medicine 3(1): 1-16.
Liu, Y., Liu, L., Mo, Y., Wei, C., Lv, L., and Luo, P. 2012. Antioxidant activity of longan (Dimocarpus longan) barks and leaves. African Journal of Biotechnology. 11(27): 7038-7045.
Povichit, N., Phrutivorapongkul, A., Suttajit, M., Chaiyasut, C. and Leelapornpisid, P. 2010. Phenolic content and in vitro inhibitory effects on oxidation and protein glycation of some Thai medicinal plants. Pakistan Journal of Pharmaceutical Sciences. 23(4): 403-408.
Pillaiyar, T., Manickam, M. and Namasivayam,V. 2017. Skin whitening agents: Medicinal chemistry perspective of tyrosinase inhibitors. Journal of Enzyme Inhibition and Medicinal Chemistry. 32(1): 403-425.
Reddy, V.P., Garrett, M.R., Perry, G., and Smith, M.A. 2005. Carnosine: A versatile antioxidant and antiglycating agent. Science of Aging Knowledge Environment. 18: pe12-pe12.
Ripa, F.A., Haque, M., and Bulbul, I.J. 2010. In vitro antibacterial, cytotoxic and antioxidant of plant Nephelium longan. Pakistan Journal of Biological Science. 13(1): 22-27.
Rivero, M.N., Lenze, M., Izaguirre, M., Damonte, S.H.P., Aguilar, A., Wikinski, S. and Gutiérrez, M.L. 2021. Comparison between HET-CAM protocols and a product use clinical study for eye irritation evaluation of personal care products, including cosmetics, according to their surfactant composition. Food and Chemical Toxicology. 153: 112229.
Schlay, S., and Slotta, U. 2016. Efficient skin protection against negative environmental influences by breathable, vegan silk polypeptides. SOFW Journal. 4: 14-17.
Theansungnoen, T., Nitthikan, N., Wilai, M., Chaiwut, P., Kiattisin, K and Intharuksa, A. 2022. Phytochemical analysis and antioxidant, antimicrobial, and anti-aging Activities of ethanolic seed extracts of four Mucuna Species. Cosmetics. 9(1): 14.
Thoo Y.Y., Ho, S.K., Liang, J.Y., Ho, C.W. and Tan, C.P. 2010. Effects of binary solvent extraction system, extraction time and extraction temperature on phenolic antioxidants and antioxidant capacity from Meng Kudu (Morinda citrifolia). Food Chemistry. 120 (1): 290-295.
Wittenauer, J., Mäckle, S., Sußmann, D., Schweiggert-Weisz, U. and Carle, R. 2015. Inhibitory effects of polyphenols from grape pomace extract on collagenase and elastase activity. Fitoterapia. 101:179-187.
Xue, Y.L., Miyakawa, T., Hayashi, Y., Okanoto, K., Hu, F., Mitani, N., Furihata, K., Sawano, Y. and Tanokura, M. 2011. Isolation and tyrosinase inhibitory effects of polyphenols from the leaves of Persimmon, Diospyros kaki. Journal of Agricultural and food Chemistry. 59(11): 6011-6017.
Xue, Y., Wang, W., Liu, Y., Zhan, R., and Chen, Y. 2015. Two new flavonol glycosides from Dimocarpus longan leaves. Natural Product Research. 29(2): 163–168.
Yeerong, K., Sriyab, S., Somwongin, S., Punyoyai, C., Chantawannakul, P., Anuchapreeda, S., Prommaban, A. and Chaiyana, W. 2021. Skin irritation and potential antioxidant, anti-collagenase, and anti-elastase activities of edible insect extracts. Scientific Reports. 11(1): 22954.
Yeh, W. J., Hsia, S. M., Lee, W. H., and Wu, C. H. 2017. Polyphenols with antiglycation activity and mechanisms of action: a review of recent findings. Journal of Food and drug Analysis. 25(1): 84–92.
Zillich, O.V., Schweiggert-Weisz, U., Eisner, P., and Kerscher, M. 2015. Polyphenol is an active ingredient in cosmetic products. International Journal of Cosmetic Science. 37(5): 455-464.
OPEN access freely available online
Natural and Life Sciences Communications
Chiang Mai University, Thailand. https://cmuj.cmu.ac.th
Pimjai Doungsaard1, Sunee Chansakaow2, Worrapan Poomanee2, 3, Jakkapan Sirithunyalug2, Nutjeera Intasai4, and Pimporn Leelapornpisid2, 3, *
1 Faculty of Pharmacy, Chiang Mai University, Chiang Mai 50200 Thailand.
2 Department of Pharmaceutical Sciences, Faculty of Pharmacy, Chiang Mai University, Chiang Mai 50200 Thailand.
3 Innovation Center for Holistic Health, Nutraceuticals and Cosmeceuticals, Faculty of Pharmacy, Chiang Mai University, Chiang Mai 50200 Thailand.
4 Division of Clinical Microscopy, Department of Medical Technology, Faculty of Associated Medical Sciences, Chiang Mai University, Chiang Mai 50200 Thailand.
Corresponding author: Pimporn Leelapornpisid E-mail: pimporn.lee@cmu.ac.th
Total Article Views
Editor: Nisit Kittipongpatana,
Chiang Mai University, Thailand
Article history:
Received: March 3, 2023;
Revised: June 11, 2023;
Accepted: June 15, 2023;
Published online: July 3, 2023

