
Clinical Effect of Virgin Coconut Oil Pulling in Comparison with Palm Oil Pulling on Gingival Health: A Crossover Randomized Clinical Controlled Trial
Nisachon Siripaiboonpong, Oranart Matangkasombut*, Haris Pengcharoen, Bongkoj Boonchaiyapluk, Phakvalunch Rujiraprasert, Pijitra Petcharat, Soranun Chantarangsu, and Supreda Suphanantachat Srithanyarat*Published Date : July 10, 2023
DOI : https://doi.org/10.12982/NLSC.2023.053
Journal Issues : Number 3, July-September 2023
Abstract The objective of this study was to compare the effects of oil pulling with virgin coconut oil (VCO), which contains antimicrobial ingredients, and with palm oil (PO) on several clinical parameters when used as adjunctive oral hygiene care in patients with gingival inflammation. In this crossover trial, thirty-six participants were randomized to group 1 to start with VCO and group 2 to start with PO pulling. The participants were instructed to continue their oral hygiene routine and to perform oil pulling by swishing 10 mL oil for 8 min for 28 days. After a 21-day wash-out period, the participants performed the protocol with the other oil type. The Gingival Index (GI), Plaque Index (PI), and salivary pH were recorded at baseline, the end of both intervention periods, and after the wash-out period. The before- and after-treatment values and the mean difference in the evaluated parameters in each group were compared. VCO pulling significantly reduced GI (P=0.004), while PO pulling significantly reduced GI (P=0.010) and PI (P=0.005) after 28 days of oil pulling. The salivary pH remained in the neutral range throughout the study period. No significant difference in salivary pH was found between the two treatments. VCO pulling did not demonstrate any significant superior effect compared with PO pulling on the evaluated clinical parameters. However, because the oil pulling interventions were not compared to negative control in this study, further studies are needed to confirm the potentially beneficial effects of oil pulling.
Keywords: Gingivitis, Oral health status, Mouth care product
Funding: This research was supported by Dental Research Fund, Dental Research Project, Faculty of Dentistry, Chulalongkorn University and Ratchadapiseksompotch Fund Chulalongkorn University to Supreda Suphanantachat Srithanyarat.
Citation: Siripaiboonpong, N., Matangkasombut, O., Pengcharoen, H., Boonchaiyapluk, B., Rujiraprasert, P., Petcharat P., Chantarangsu, S., Srithanyarat, S.S. 2023. Clinical effect of virgin coconut oil pulling in comparison with palm oil pulling on gingival health: A crossover randomized clinical controlled trial. Natural and Life Sciences Communications. 22(3): e2023053.
INTRODUCTION
In Ayurvedic medicine, oil pulling is the practice of swishing oil in the mouth, which helps in preventing and treating oral diseases (Sooryavanshi et al., 1994; Tomar et al., 2014). Currently, oil pulling is highly discussed in complementary medicine and is believed to beneficial in preventing tooth decay, bleeding gums, and malodor, as well as treating systemic problems, e.g., headache and diabetes (Tomar et al., 2014). The popularity of oil pulling has increased along with the trend of using natural products in health care (Li et al., 2022). Oil pulling can be done with many edible oils, especially sesame oil, sunflower oil, and coconut oil (Tomar et al., 2014). A study reported that an emulsion from oil pulling contained more bacteria than that of saline pulling and resulted in a significantly reduced number of bacteria in saliva samples (Griessl et al., 2021).
Coconut oil is an inexpensive and accessible household product, especially in Southeast Asia. According to the manufacturing method, there are 2 types of coconut oil; refined, bleached, and deodorized coconut oil (RBD) and virgin coconut oil (VCO). VCO undergoes a special extraction process by extracting the oil directly from fresh coconut flesh or coconut milk without using high temperature in contrast to the RBD coconut oil method (Nevin et al., 2004). Unlike other types of vegetable oil that contain long-chain fatty acids, the predominant fatty acid found in VCO is a medium-chain fatty acid called Lauric acid (55.4– 59.1%) (Kurata et al., 2005). Most of the fatty acids contained in VCO are triglycerides (84–93.1%) and the remaining are diglycerides, monoglycerides, and free fatty acids (Gopalakrishnan et al., 1987; Dumancas et al., 2016). In the oil pulling process, more free fatty acids and monoglycerides are generated by the lipolytic activity of lipase in the saliva (Neyraud et al., 2012; Voigt et al., 2014; Lai et al., 2019). Lauric acid and its derivative monolaurin have demonstrated antimicrobial effects by disrupting bacterial and viral cells as well as inhibiting signal transduction and transcription in the microorganisms (Dayrit, 2015). However, in our previous study, which evaluated the microbiological effect of VCO and palm oil (PO) pulling, no significant reductions in the number of aerobic or anaerobic microorganisms were found in VCO pulling group. In that study, only a significant reduction in mutans streptococci was observed after PO pulling (Siripaiboonpong et al., 2022).
Although previous in-vitro and clinical studies on the effectiveness of coconut oil pulling found positive outcomes on oral microorganisms, gingival health, and plaque control (Peedikayil et al., 2015; Chalke et al., 2017; Nagilla et al., 2017; Kaliamoorthy et al., 2018; Kaliamoorthy et al., 2018; Sezgin et al., 2019; Menaka et al., 2020; Chanpa et al., 2023), the current systematic reviews have reported that there is no high-quality data and more rigorous scientific evidence is required to support the benefit of coconut oil pulling (Woolley et al., 2020; Raja et al., 2021).
Therefore, the objective of this study was to compare the effect of VCO pulling with PO pulling on specific clinical parameters when used as an adjunctive oral hygiene care method in patients with gingival inflammation. The Gingival Index (GI) was the primary outcome, and the Plaque Index (PI) and salivary pH were the secondary outcomes.
MATERIALS AND METHODS
Clinical trial design
This randomized controlled trial (Thai Clinical Trial Registry No: TCTR20180515003) was designed to compare the effect of VCO pulling with PO pulling on specific clinical parameters using a crossover design. The study protocol was approved by the Human Research Ethics Committee of the Faculty of Dentistry, Chulalongkorn University (HRE-DCU 2018-007) and was conducted in accordance with the Helsinki Declaration as revised in 2013. The primary outcome of the study was to determine whether gingival inflammation could be reduced by performing VCO pulling compared with PO pulling. The secondary outcomes were the effect of VCO and PO pulling on plaque accumulation and salivary pH.
Participants
The periodontal screening examinations were performed by experienced periodontists at the Department of Periodontology, Faculty of Dentistry, Chulalongkorn University. Thirty-six adults, who were diagnosed with gingivitis as assessed using the GI ≥1 (Löe et al., 1963), were recruited in the study. All participants provided informed consent and agreed to refrain from dental treatment during the study period. The data reported by Anand et al. (Anand et al., 2008) were used to calculate the sample size using the following formula in n4Studies application. An additional 10% was added to the calculated sample size to compensate for potential drop-outs.
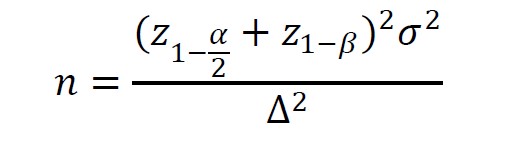
σ = SD of the data = 0.4; ∆ = difference of data between 2 groups = 0.2
α = 0.05, Z (0.975) = 1.959964; β = 0.20, Z (0.8000) = 0.841621
Sample size from formula = 32; Actual sample size (n) = 36
The participants who were allergic to oil, undergoing orthodontic treatment, smokers, using mouthwash, or had a history of systemic diseases were excluded from the study.
Randomization and blinding
The subject allocation and randomization was performed as previously described (Siripaiboonpong et al., 2022). Briefly, the participants were allocated into two groups: (1) starting with VCO (test) or (2) starting with PO (control) using block randomization. To lower the bias due to multiple examiners, each type of measurement was performed by 1 calibrated examiner who was blinded to the treatment group of the participants; PI (P.R.), GI (B.B.), and salivary pH (H.P.). The intra-examiner calibration was performed by re-examining three subjects after a two-hour interval and the Intraclass Correlation Coefficient (ICC) was acceptable at > 0.75 (Koo et al., 2016).
Intervention
VCO (Parisut®, Mada Miracle Co.,LTD., Bangkok, Thailand), cold pressed coconut oil, was used in the test group. PO (Oleen®, Oleen Co.,LTD., Samut Sakorn, Thailand) was used in the control group because it does not have the active ingredient (lauric acid) and has a similar viscosity to that of VCO. The fatty acid composition of each oil was assessed by Gas Chromatograph-Mass Spectrometer/Mass Spectrometer as previously reported (Siripaiboonpong et al., 2022).
The oil pulling procedure comprised swishing 10 mL oil in the oral cavity for 8 min and spitting out the liquid (Amith et al., 2007). The procedure was performed daily in the morning after the participants’ routine oral hygiene care for 28 d. The participants then entered a wash-out period for 21 d. During this time, the participants performed only their routine oral hygiene care. Next, the procedure was repeated with the other oil type for 28 d (Figure 1). The subjects were interviewed and completed a questionnaire to determine their compliance. The participants were also asked to return the empty bottles of the assigned oil.
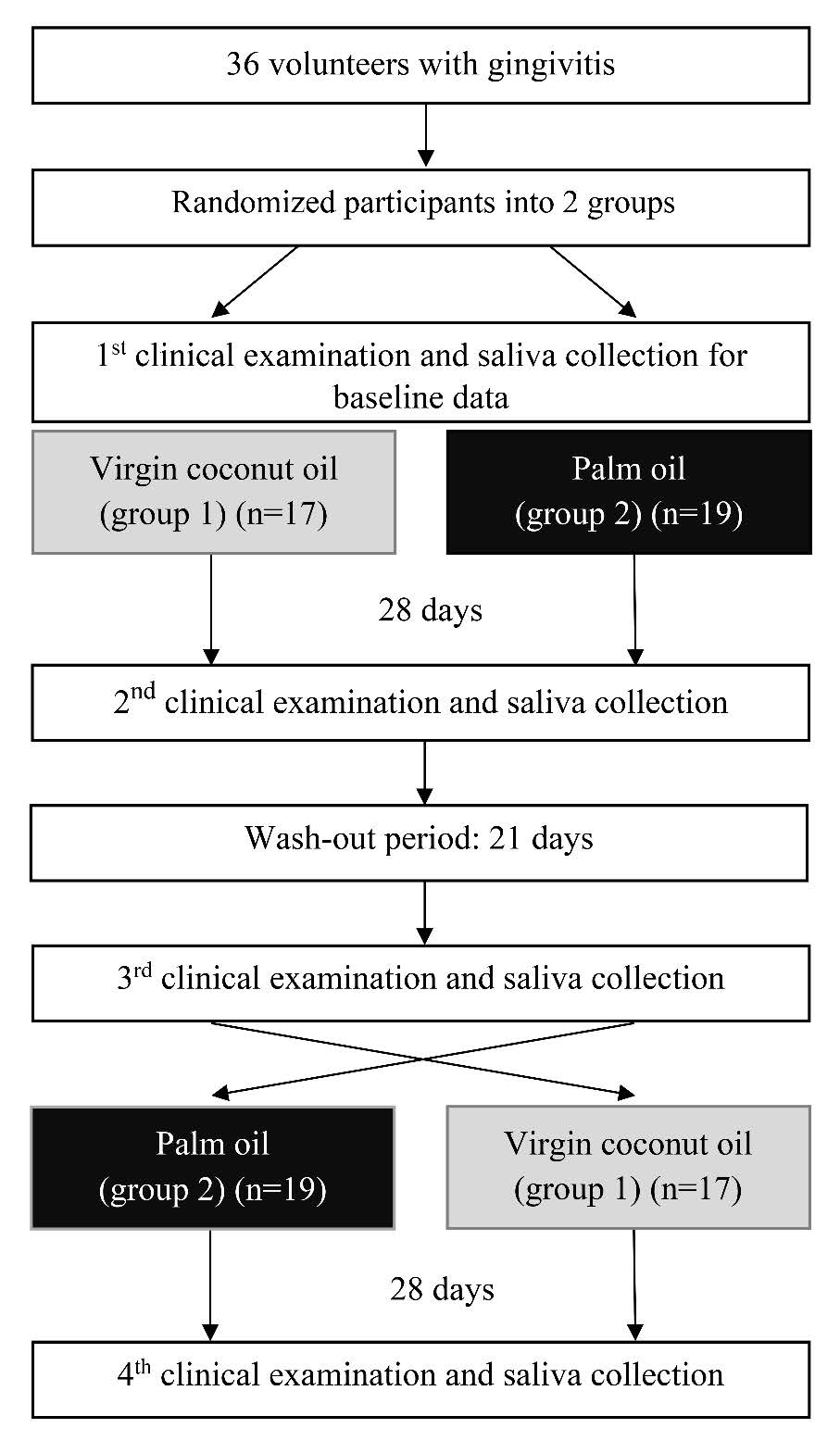
Figure 1. Flow chart of the study design
The participants were assessed for the GI, (Löe et al., 1963) PI, (Turesky et al., 1970) and salivary pH at baseline, Day 28 of the first intervention period, after the wash-out period, and Day 28 of the second intervention period (Figure 1). At baseline, the particpants’ demographic data and history of their previous periodontal treatment, medical history, and oral hygiene practice were obtained by interviews.
Outcome measurements
Although the primary outcome of this study was the participants’ gingival health status assessed using GI, the PI was scored prior to scoring the GI to minimize disturbing the dental plaque biofilm while probing. The modified Quigley- Hein Index (PI) was scored by inspecting the quantity of plaque accumulated at the labial/buccal and lingual/palatal surfaces of teeth 16, 12, 24, 36, 32, and 44 using unaided eyes without any auxiliary equipment, and recording the scores according to the criteria described by Turesky et al. (Turesky et al., 1970). If a designated tooth was missing, the adjacent tooth was used instead. The average score of each participant was calculated by dividing the sum of the scores by the total surface number.
Gingival health was assessed using the Löe-Silness GI (Löe et al., 1963). The GI was assessed after PI recording at the same teeth by probing at 4 sites (mesiobuccal/labial, mid-buccal/labial, distobuccal/labial, and mid-lingual/palatal) of each tooth using a UNC-15 periodontal probe. The GI score of each tooth was determined by calculating the sum of the total scores and dividing by four. Next, the mean values of the examined teeth were used as the GI score of each subject.
The salivary pH was measured from stimulated saliva collected from each participant prior to any examination on the data collection days. Each participant chewed on a piece of paraffin for 5 min and spitted the saliva into a sterilized container. The salivary pH was measured using a pH meter (Orion model 420A, Thermo Electron Corporation, MA, USA). The pH meter was calibrated before each pH measurement according to the manufacturer’s instruction. Each sample was measured 3 times and the average pH was calculated.
Statistical analysis
The effect of VCO and PO interventions over time on the clinical parameters and their interactions were evaluated using two-way repeated measures ANOVA followed by Least Significant Difference (LSD) post-hoc analysis. The mean changes (before – after the intervention) of each clinical parameters between VCO and PO interventions were compared using the paired t-test for PI and salivary pH and Wilcoxon signed-rank test for GI due to skewed data. A P-value < 0.05 was considered significant. The SPSS statistics (SPSS version 29.0, SPSS Inc., IL, USA) software was used for all statistical analyses.
RESULTS
Demographic data and oral hygiene habits of participants
Thirty-six participants completed the study. The detailed participants’ characteristics were previously reported (Siripaiboonpong et al., 2022). Briefly, the participants were 19–29 years old (mean 23.4 years old) and 25 (69.4%) were female. Most of the participants brushed their teeth twice a day (86.1%) using the modified Bass technique (88.9%). In contrast, approximately half of the participants performed regular interdental cleansing (47.2%).
Comparison of outcome measurements
Two-way repeated measures ANOVA demonstrated no statistically significant effect of the interventions on the clinical parameters including GI, PI, and salivary pH (P = 0.500, 0.558, and 0.650, respectively) and no interaction between interventions and times on all clinical parameters (P = 0.650, 0.060, and 0.345, respectively). While there was a statistically significant effect of times on GI, PI, and salivary pH (P < 0.001, 0.010, and 0.038, respectively). (Table 1).
The mean baseline value of the GI, PI, and salivary pH between VCO and PO groups were not statistically significantly different (P = 0.895, 0.172, and 0.380, respectively). Similarly, the GI, PI, and salivary pH after oil pulling and their mean changes (before – after) showed no statistically significant difference between VCO and PO groups (P > 0.05) (Table 2). The GI and PI scores at baseline and after the 28-day period of oil pulling with VCO and with PO are shown in Figure 2a and 2b, respectively. After PO pulling, significant reductions in the GI (P = 0.010) and PI scores (P = 0.005) from baseline were observed. However, in the VCO pulling group, a significant reduction was observed for the GI score (P = 0.004), but not the PI score (P = 0.126). The salivary pH ranged from 6.98–8.16 before and after the intervention. The mean salivary pH before and after PO pulling were 7.62 ± 0.21 and 7.52 ± 0.28 (P = 0.045), whereas the mean salivary pH before and after VCO pulling were 7.57 ± 0.21 and 7.52 ± 0.26 (P = 0.171), respectively (Figure 2c). Thus, a significant change in salivary pH was observed in the PO pulling group.
Table 1. Two-way repeated measures ANOVA for treatments (VCO and PO) and times (before and after oil pulling) on the clinical parameters including Gingival Index, Plaque Index and Salivary pH.
|
Source |
df |
Mean Squares |
Partial Eta Squared |
F |
P-value |
|
Gingival Index |
|||||
|
Interventions |
1 |
0.012 |
0.013 |
0.464 |
0.500 |
|
Times |
1 |
0.522 |
0.341 |
18.144 |
<0.001 |
|
Treatments x Times |
1 |
0.007 |
0.006 |
0.210 |
0.650 |
|
Plaque Index |
|||||
|
Interventions |
1 |
0.041 |
0.010 |
0.350 |
0.558 |
|
Times |
1 |
1.100 |
0.173 |
7.317 |
0.010 |
|
Treatments x Times |
1 |
0.179 |
0.097 |
3.770 |
0.060 |
|
Salivary pH |
|||||
|
Interventions |
1 |
0.017 |
0.006 |
0.209 |
0.650 |
|
Times |
1 |
0.208 |
0.118 |
4.662 |
0.038 |
|
Treatments x Times |
1 |
0.020 |
0.026 |
0.916 |
0.345 |
Note: Statistically significant difference (P < 0.05) indicated in bold.
Table 2. Comparison of the clinical parameters including Gingival Index, Plaque Index and Salivary pH from different treatments (VCO and PO) at different time points (before and after oil pulling) and mean changes over time.
|
Parameters |
Virgin coconut oil (Mean ± SD) |
Palm oil (Mean ± SD) |
P-value (VCO VS PO) |
|
Gingival Index |
|
|
|
|
Baseline |
1.50 ± 0.12 |
1.50 ± 0.16 |
0.895a |
|
After oil pulling |
1.37 ± 0.23 |
1.40 ± 0.21 |
0.485a |
|
Mean changes (Before – After) |
0.13 ± 0.26 |
0.11 ± 0.24 |
0.508b |
|
P-value (Baseline VS After) |
0.004a |
0.010a |
|
|
Plaque Index |
|
|
|
|
Baseline |
1.04 ± 0.33 |
1.15 ± 0.40 |
0.172a |
|
After oil pulling |
0.94 ± 0.28 |
0.90 ± 0.29 |
0.535a |
|
Mean changes (Before – After) |
0.10 ± 0.40 |
0.25 ± 0.49 |
0.060c |
|
P-value (Baseline VS After) |
0.126a |
0.005a |
|
|
Salivary pH |
|
|
|
|
Baseline |
7.57 ± 0.21 |
7.62 ± 0.21 |
0.380a |
|
After oil pulling |
7.52 ± 0.26 |
7.52 ± 0.28 |
0.975a |
|
Mean changes (Before – After) |
0.05 ± 0.22 |
0.10 ± 0.29 |
0.345c |
|
P-value (Baseline VS After) |
0.171a |
0.045a |
|
Note: aTwo-way repeated measures ANOVA followed by Least Significant Difference (LSD) post-hoc analysis.; bWilcoxon signed-rank test.; cPaired t-test.; Statistically significant difference (P < 0.05) indicated in bold.
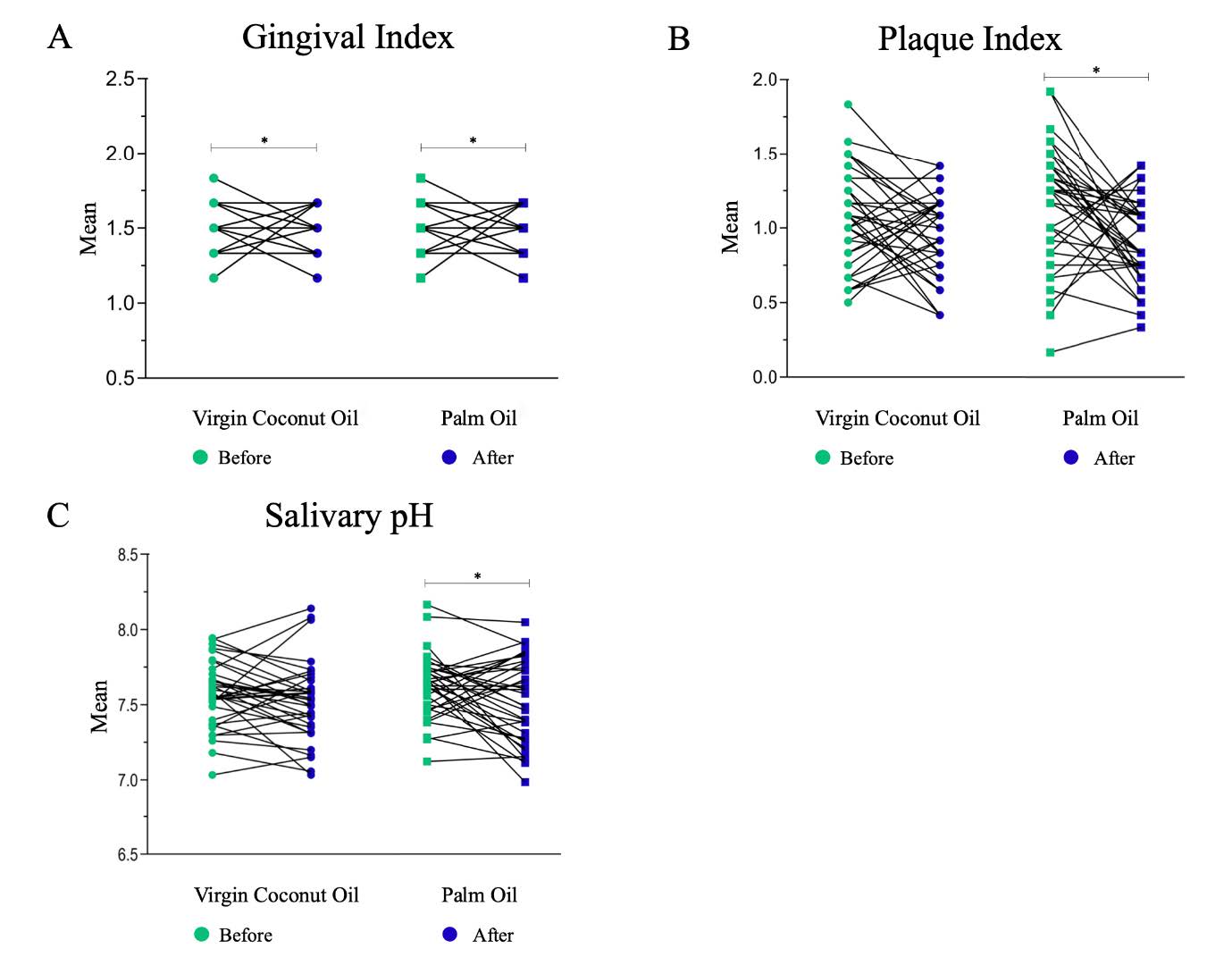
Figure 2. Mean score of the clinical parameters before and after oil pulling (A), Gingival Index (GI); (B), Plaque Index (PI); and (C), Salivary pH.
*Significant reduction in the mean score from baseline, analyzed by two-way repeated measures ANOVA followed by Least Significant Difference (LSD) post-hoc analysis (P < 0.05).
DISCUSSION
The present randomized controlled trial compared the effects of VCO pulling with PO pulling when used as an adjunctive oral hygiene practice on clinical parameters comprising GI score, PI score, and salivary pH. VCO contains abundant antimicrobial components (lauric acid and monolaurin), while the amount of active ingredients in PO was very low (Siripaiboonpong et al., 2022). Furthermore, VCO and PO have similar physical properties, which allowed us to focus on the effect of the active ingredients. We found no significant difference in the mean changes in all parameters between VCO pulling and PO pulling. Thus, the antimicrobial components of VCO did not demonstrate a significant benefit on reducing plaque or gingival inflammation. However, a significant reduction in the GI, PI, and salivary pH after oil pulling compared to baseline was found. Nonetheless, VCO pulling only significantly lowered the GI value. This suggests that PO pulling might benefit gingival health and can be considered as an adjunctive oral hygiene routine. However, additional studies on the clinical effect and mechanism of PO pulling, especially clinical comparison with a negative control or placebo group, are needed to confirm these results.
Although limited clinical evidence exists on the effects of coconut oil pulling, similar positive results were reported for GI, but not for PI and salivary pH (Peedikayil et al., 2015; Peedikayil et al., 2015; Chalke et al., 2017; Kaliamoorthy et al., 2018; Menaka et al., 2020). However, these studies had certain limitations and their methodologies differed from our study. Most of the studies used oil pulling as an adjunct to normal oral hygiene practice. Two studies reported a significant reduction in PI and modified GI scores after 7–30 days (Peedikayil et al., 2015; Chalke et al., 2017), however, no control group was included, thus the Hawthorne effect could not be ruled out. A randomized controlled trial compared the effect of coconut oil pulling when used adjunctively with toothbrushing to toothbrushing alone. The results demonstrated a significant reduction in GI and PI in the test group and a significant difference between the two groups (Menaka et al., 2020). However, using brushing only as the control group could not indicate whether the effects resulted from the process of oil pulling or the active ingredients of coconut oil. To address this research gap, our study was designed to compare the clinical effects between VCO and PO pulling.
Previous studies have demonstrated that acidic saliva correlates with periodontitis and dental caries, however, the correlation between salivary pH and gingivitis is inconclusive (Holbrook et al., 1993; Galgut, 2001; Baliga et al., 2013; Cunha-Cruz et al., 2013; Bansal et al., 2016). Although our results revealed that PO pulling significantly lowered the mean salivary pH from 7.62 to 7.52, and VCO pulling reduced the pH from 7.57 to 7.52, the pH values were within the neutral range.
Despite the absence of the active ingredient (lauric acid), PO pulling demonstrated a beneficial effect on the GI, PI, and salivary pH, while VCO pulling only reduced the GI compared with baseline. We speculate that in addition to the active ingredients, the process of oil pulling contributed to the reduced GI and PI scores.
Although the mechanism of oil pulling in reducing plaque and improving gingival health has not been scientifically explained, some hypotheses have been proposed. First, oil pulling increased the hydrophobicity of the acquired pellicle by forming a lipid-enriched pellicle on the tooth surface that prevented bacterial adhesion (Kensche et al., 2013). This hypothesis was supported by a study that used a direct visualization method and found nano- and micro-sized lipid droplets attached on the tooth surfaces after rinsing with oil for 30 sec. The lipid droplets remained adhered for several hours (Peckys et al., 2019). Moreover, it was postulated that the force generated during the pulling action initiates emulsification between the oil and saliva, which increased the viscosity of the saliva (Griessl et al., 2021). Lipid micelles in the emulsion might interact with bacteria-containing epithelial cells via lecithin and glycerol and resulted in encapsulating the cells (Griessl et al., 2021). The lengthy period of pulling and the increased viscosity might play a part in the transient reduction in microbial burden after oil pulling. Microscopic analysis revealed infected epithelial cells surrounded by lipid droplets after oil pulling (Griessl et al., 2021). The higher viscosity of PO could contribute to its superior plaque reduction effect compared with VCO (Siddiqui et al., 2013).
Among chemical plaque control measures, Chlorhexidine gluconate is considered the most effective antiplaque and antimicrobial mouthwash. However, due to its adverse effects, including staining and taste alteration, it is not recommended for everyday use (Flötra et al., 1971). Oil pulling, along with other natural remedies, is believed to have less adverse effects and can be practiced adjunctively to routine oral hygiene practice. In our study, the participants reported no adverse effects from either type of oil pulling. A previous study reported a similar plaque inhibitory effect of coconut oil pulling and chlorhexidine mouthwash (Sezgin et al., 2019).
The crossover design of our study enabled the comparison of the two interventions in the same participants and also helped to control the variations of contributing factors between them. The variations in brushing techniques, brushing frequency, and interdental cleansing were controlled because they were compared within the same individuals. The mean difference in each parameter was used to compare the effect of VCO and PO pulling.
This study has some potential limitations. First, the true compliance of the participants could not be confirmed. In this study, the oil pulling process took 8 min (Amith et al., 2007) which is relatively long and could negatively affect the participants’ compliance. We evaluated their compliance by interviewing them and checking the returned empty oil bottles, which demonstrated a comparable level of compliance as reported in our previous study. Second, only participants with gingivitis were included in the study. It would be beneficial for further studies to evaluate the effect of oil pulling on periodontitis patients. Lastly, because our study did not have a negative control, the Hawthorne effect could not be ruled out. Therefore, the positive effect of PO pulling should be confirmed in larger trials using a placebo or mechanical cleaning as the control group.
CONCLUSION
Compared with PO pulling, VCO pulling has no superior effect on gingival health. Performing oil pulling with VCO or PO as an adjunctive oral hygiene routine may benefit gingival health. However, because the oil pulling interventions were not compared to negative control in this study, further studies are needed to confirm the potentially beneficial effects of oil pulling.
ACKNOWLEDGMENTS
The authors greatly appreciate to valuable advices given by Professor Emeritus Niklaus P. Lang, University of Berne, Switzerland and Professor Piyamitr Sritara, Faculty of Medicine, Ramathibodi Hospital, Thailand. Moreover, we acknowledge the advice from Associate Professor Dr. Waranuch Pitiphat, Faculty of Dentistry, Khon Kaen University, Thailand; Dr. Suthee Rattanamongkolgul, Faculty of Medicine, Srinakharinwirot University Thailand on statistical analysis. We also thank the staff of the periodontal post-graduate clinic for their clinical assistances. Lastly, we thank Dr. Kevin Tompkins for his assistance in English language editing of the manuscript.
AUTHOR CONTRIBUTIONS
Supreda Suphanantachat Srithanyarat conceived the ideas; Supreda Suphanantachat Srithanyarat and Oranart Matangkasombut designed the study; Haris Pengcharoen, Bongkoj Boonchaiyapluk, and Phakvalunch Rujiraprasert collected the clinical data; Pijitra Petcharat and Soranun Chantarangsu analyzed the data; Nisachon Siripaiboonpong collected microbiological data, analysed the data and drafted the manuscript; and Supreda Suphanantachat Srithanyarat and Oranart Matangkasombut finalized the manuscript. All authors read and approved the final manuscript.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
REFERENCES
Amith, H., Ankola, A. V. and Nagesh, L. 2007. Effect of oil pulling on plaque and gingivitis. Journal of Oral Health and Community Dentistry. 1(1): 12-18.
Anand, T. D., Pothiraj, C., Gopinath, R. and Kayalvizhi, B. 2008. Effect of oil-pulling on dental caries causing bacteria. African Journal of Microbiology Research. 2(3): 63-66.
Baliga, S., Muglikar, S. and Kale, R. 2013. Salivary pH: A diagnostic biomarker. Journal of Indian Society of Periodontology. 17(4): 461.
Bansal, D. K., Khuller, N., Bansal, P., Bhatia, A. and Mehta, A. 2016. Role of salivary flow rate and salivary ph as a diagnostic marker in smokers with chronic periodontitis. Indian Journal of Stomatology. 7(1): 19-22.
Chalke, S., Zope, S., Suragimath, G., Varma, S., Abbayya, K. and Kale, V. 2017. Effect of coconut oil pulling on plaque-induced gingivitis: A prospective clinical study. International Journal of Green Pharmacy. 11(4): 750-755.
Chanpa, P., Owittayakul, D., Wanachantararak, P., Chaiyana, W. and Sookkhee, S. 2023. Formulation of coconut oil mouthwash with mixed emulsifier and its growth inhibition of Candida albicans biofilms. Chiang Mai University Journal of Natural Sciences 22(1): 1-16.
Cunha-Cruz, J., Scott, J., Rothen, M., Mancl, L., Lawhorn, T., Brossel, K., Berg, J. and DENTistry, N. P.-b. R. C. i. E.-b. 2013. Salivary characteristics and dental caries: evidence from general dental practices. The Journal of the American Dental Association. 144(5): e31-e40.
Dayrit, F. M. 2015. The properties of lauric acid and their significance in coconut oil. Journal of the American Oil Chemists Society. 92(1): 1-15.
Dumancas, G. G., Viswanath, L. C. K., de Leon, A. R., Ramasahayam, S., Maples, R., Koralege, R. H., Perera, U. D. N., Langford, J., Shakir, A. and Castles, S. 2016. Health benefits of virgin coconut oil. Vegetable Oil: Properties, Uses and Benefits. B. Holt. Burleigh, Australia, NOVA Science Publishers, Inc.: 161-194.
Flötra, L., Gjermo, P., Rölla, G. and Waerhaug, J. 1971. Side effects of chlorhexidine mouth washes. European Journal of Oral Sciences. 79(2): 119- 125.
Galgut, P. 2001. The relevance of pH to gingivitis and periodontitis. Journal of the International Academy of Periodontology. 3(3): 61-67.
Gopalakrishnan, N., Narayanan, C., Mathew, A. and Arumughan, C. 1987. Lipid composition of coconut cake oil. Journal of the American Oil Chemists Society. 64(4): 539-541.
Griessl, T., Zechel-Gran, S., Olejniczak, S., Weigel, M., Hain, T. and Domann, E. 2021. High-resolution taxonomic examination of the oral microbiome after oil pulling with standardized sunflower seed oil and healthy participants: A pilot study. Clinical Oral Investigations. 25(5): 2689-2703.
Holbrook, W., De Soet, J. and De Graaff, J. 1993. Prediction of dental caries in pre-school children. Caries Research. 27(5): 424-430.
Kaliamoorthy, S., Pazhani, A., Nagarajan, M., Meyyappan, A., Rayar, S. and Mathivanan, S. 2018. Comparing the effect of coconut oil pulling practice with oil pulling using sesame oil in plaque-induced gingivitis: A prospective comparative interventional study. Journal of Natural Science, Biology, and Medicine. 9(2): 165-168.
Kaliamoorthy, S., Vijayakumar, J., Caliaperoumal, S. K., Pazhani, A., Raju, K., Venkatesan, P. and Murugaboopathy, V. 2018. Comparing the effect of coconut oil pulling practice with chlorhexidine mouth wash in plaque induced gingivitis by evaluation of salivary biochemical marker–a comparative interventional study. Journal of Natural Remedies. 18(4): 151-155.
Kensche, A., Reich, M., Kümmerer, K., Hannig, M. and Hannig, C. 2013. Lipids in preventive dentistry. Clinical oral investigations. 17(3): 669-685.
Koo, T. K. and Li, M. Y. 2016. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. Journal of Chiropractic Medicine. 15(2): 155-163.
Kurata, S., Yamaguchi, K. and Nagai, M. 2005. Rapid discrimination of fatty acid composition in fats and oils by electrospray ionization mass spectrometry. Analytical sciences. 21(12): 1457-1465.
Lai, W. Y. W., Chua, J. W. M., Gill, S. and Brownlee, I. A. 2019. Analysis of the lipolytic activity of whole-saliva and site-specific secretions from the oral cavity of healthy adults. Nutrients. 11(1): 191.
Li, M., Kamdenlek, P., Kuntanawat, P., Eawsakul, K., Porntaveetus, T., Osathanon, T. and Manaspon, C. 2022. In vitro preparation and evaluation of chitosan/pluronic f-127 hydrogel as a local delivery of crude extract of phycocyanin for treating gingivitis. Chiang Mai University Journal of Natural Sciences 21(4): 1-13.
Löe, H. and Silness, J. 1963. Periodontal disease in pregnancy I. Prevalence and severity. Acta Odontologica Scandinavica. 21(6): 533-551.
Menaka, V., Kavya, G., Bhuvaneshwari, R., Azali, A., Aparna, S. and Kumar, P. 2020. Effectiveness of coconut oil pulling as an adjuvant to oral hygiene procedure on plaque-induced gingivitis among middle-aged adults – An interventional study. Journal of Global Oral Health. 2: 102-107.
Nagilla, J., Kulkarni, S., Madupu, P. R., Doshi, D., Bandari, S. R. and Srilatha, A. 2017. Comparative evaluation of antiplaque efficacy of coconut oil pulling and a placebo, among dental college students: A randomized controlled trial. Journal of clinical and diagnostic research: Journal of Clinical and Diagnostic Research. 11(9): ZC08.
Nevin, K. and Rajamohan, T. 2004. Beneficial effects of virgin coconut oil on lipid parameters and in vitro LDL oxidation. Clinical Biochemistry. 37(9): 830- 835.
Neyraud, E., Palicki, O., Schwartz, C., Nicklaus, S. and Feron, G. 2012. Variability of human saliva composition: Possible relationships with fat perception and liking. Archives of Oral Biology. 57(5): 556-566.
Peckys, D., De Jonge, N. and Hannig, M. 2019. Oil droplet formation on pellicle covered tooth surfaces studied with environmental scanning electron microscopy. Journal of Microscopy. 274(3): 158-167.
Peedikayil, F. C., Sreenivasan, P. and Narayanan, A. 2015. Effect of coconut oil in plaque related gingivitis—A preliminary report. Nigerian Medical Journal. 56(2): 143.
Peedikayil, F. C., Sreenivasan, P. and Narayanan, A. 2015. Effect of coconut oil in plaque related gingivitis—A preliminary report. Nigerian Medical Journal. 56(2): 143-147.
Raja, B. K. and Devi, K. 2021. Oral health effects of oil pulling: A systematic review of randomized controlled trials. Journal of Indian Association of Public Health Dentistry. 19(3): 170.
Sezgin, Y., Ozgul, B. M. and Alptekin, N. O. 2019. Efficacy of oil pulling therapy with coconut oil on four-day supragingival plaque growth: A randomized crossover clinical trial. Complementary Therapies in Medicine. 47: 102193.
Siddiqui, N. and Ahmad, A. 2013. A study on viscosity, surface tension and volume flow rate of some edible and medicinal oils. International Journal of Environmental Science and Technology. 2(6): 1318-1326.
Siripaiboonpong, N., Matangkasombut, O., Pengcharoen, H., Boonchaiyapluk, B., Rujiraprasert, P. and Srithanyarat, S. S. 2022. Microbiological effects of virgin coconut oil pulling in comparison with palm oil pulling as an adjunctive oral hygiene care for patients with gingival inflammation: A randomized controlled clinical trial. Journal of Indian Society of Periodontology. 26(1): 58.
Sooryavanshi, S. and Mardikar, B. 1994. Prevention and treatment of diseases of mouth by gandoosha and kavala. Ancient Science of Life. 13(3-4):266-270.
Tomar, P., Hongal, S., Jain, M., Rana, K. and Saxena, V. 2014. Oil pulling and oral health: A review. International Journal of Scientific Study. 1(3): 33-37.
Turesky, S., Gilmore, N. D. and Glickman, I. 1970. Reduced plaque formation by the chloromethyl analogue of victamine C. Journal of Periodontology. 41(1): 41-43.
Voigt, N., Stein, J., Galindo, M. M., Dunkel, A., Raguse, J.-D., Meyerhof, W., Hofmann, T. and Behrens, M. 2014. The role of lipolysis in human orosensory fat perception. Journal of Lipid Research. 55(5): 870-882.
Woolley, J., Gibbons, T., Patel, K. and Sacco, R. 2020. The effect of oil pulling with coconut oil to improve dental hygiene and oral health: A systematic review. Heliyon. 6(8): e04789.
OPEN access freely available online
Natural and Life Sciences Communications
Chiang Mai University, Thailand. https://cmuj.cmu.ac.th
Supplement
 CONSORT 2010 checklist of information to include when reporting a randomised trial*
CONSORT 2010 checklist of information to include when reporting a randomised trial*
|
Section/Topic |
Item No |
Checklist item |
Reported on page No |
||
|
Title and abstract |
|||||
|
|
1a |
Identification as a randomised trial in the title |
1 |
||
|
1b |
Structured summary of trial design, methods, results, and conclusions (for specific guidance see CONSORT for abstracts) |
1 |
|||
|
Introduction |
|||||
|
Background and objectives |
2a |
Scientific background and explanation of rationale |
2-3 |
||
|
2b |
Specific objectives or hypotheses |
3 |
|||
|
Methods |
|||||
|
Trial design |
3a |
Description of trial design (such as parallel, factorial) including allocation ratio |
3-5 |
||
|
3b |
Important changes to methods after trial commencement (such as eligibility criteria), with reasons |
- |
|||
|
Participants |
4a |
Eligibility criteria for participants |
4 |
||
|
4b |
Settings and locations where the data were collected |
5 |
|||
|
Interventions |
5 |
The interventions for each group with sufficient details to allow replication, including how and when they were actually administered |
5 |
||
|
Outcomes |
6a |
Completely defined pre-specified primary and secondary outcome measures, including how and when they were assessed |
3, 7 |
||
|
6b |
Any changes to trial outcomes after the trial commenced, with reasons |
- |
|||
|
Sample size |
7a |
How sample size was determined |
- |
||
|
7b |
When applicable, explanation of any interim analyses and stopping guidelines |
- |
|||
|
Randomisation: |
|
|
|
||
|
Sequence generation |
8a |
Method used to generate the random allocation sequence |
4-5 |
||
|
8b |
Type of randomisation; details of any restriction (such as blocking and block size) |
5 |
|||
|
Allocation concealment mechanism |
9 |
Mechanism used to implement the random allocation sequence (such as sequentially numbered containers), describing any steps taken to conceal the sequence until interventions were assigned |
- |
||
|
Implementation |
10 |
Who generated the random allocation sequence, who enrolled participants, and who assigned participants to interventions |
4-5 |
||
|
Blinding |
11a |
If done, who was blinded after assignment to interventions (for example, participants, care providers, those assessing outcomes) and how |
5 |
||
|
11b |
If relevant, description of the similarity of interventions |
- |
|||
|
Statistical methods |
12a |
Statistical methods used to compare groups for primary and secondary outcomes |
8 |
||
|
12b |
Methods for additional analyses, such as subgroup analyses and adjusted analyses |
- |
|||
|
Results |
|||||
|
Participant flow (a diagram is strongly recommended) |
13a |
For each group, the numbers of participants who were randomly assigned, received intended treatment, and were analysed for the primary outcome |
8 |
||
|
13b |
For each group, losses and exclusions after randomisation, together with reasons |
- |
|||
|
Recruitment |
14a |
Dates defining the periods of recruitment and follow-up |
- |
||
|
14b |
Why the trial ended or was stopped |
- |
|||
|
Baseline data |
15 |
A table showing baseline demographic and clinical characteristics for each group |
9, Table 2 |
||
|
Numbers analysed |
16 |
For each group, number of participants (denominator) included in each analysis and whether the analysis was by original assigned groups |
9, Figure 1 |
||
|
Outcomes and estimation |
17a |
For each primary and secondary outcome, results for each group, and the estimated effect size and its precision (such as 95% confidence interval) |
8-9, Table 1, Figure 2 |
||
|
17b |
For binary outcomes, presentation of both absolute and relative effect sizes is recommended |
- |
|||
|
Ancillary analyses |
18 |
Results of any other analyses performed, including subgroup analyses and adjusted analyses, distinguishing pre-specified from exploratory |
- |
||
|
Harms |
19 |
All important harms or unintended effects in each group (for specific guidance see CONSORT for harms) |
- |
||
|
Discussion |
|||||
|
Limitations |
20 |
Trial limitations, addressing sources of potential bias, imprecision, and, if relevant, multiplicity of analyses |
14 |
||
|
Generalisability |
21 |
Generalisability (external validity, applicability) of the trial findings |
15 |
||
|
Interpretation |
22 |
Interpretation consistent with results, balancing benefits and harms, and considering other relevant evidence |
11-13 |
||
|
Other information |
|
||||
|
Registration |
23 |
Registration number and name of trial registry |
3 |
||
|
Protocol |
24 |
Where the full trial protocol can be accessed, if available |
Figure 1 |
||
|
Funding |
25 |
Sources of funding and other support (such as supply of drugs), role of funders |
Acknowledgements |
||
*We strongly recommend reading this statement in conjunction with the CONSORT 2010 Explanation and Elaboration for important clarifications on all the items. If relevant, we also recommend reading CONSORT extensions for cluster randomised trials, non-inferiority and equivalence trials, non- pharmacological treatments, herbal interventions, and pragmatic trials. Additional extensions are forthcoming: for those and for up to date references relevant to this checklist, see www.consort-statement.org.
Nisachon Siripaiboonpong1, 2, Oranart Matangkasombut 3, 4, *, Haris Pengcharoen5, Bongkoj Boonchaiyapluk5, Phakvalunch Rujiraprasert5, Pijitra Petcharat6, Soranun Chantarangsu7, and Supreda Suphanantachat Srithanyarat1, 2, *
1 Department of Periodontology, Faculty of Dentistry, Chulalongkorn University, Bangkok, Thailand.
2 Center of Excellence in Periodontal Disease and Dental Implant, Faculty of Dentistry, Chulalongkorn University, Bangkok, Thailand.
3 Department of Microbiology and Research Unit on Oral Microbiology and Immunology, Faculty of Dentistry, Chulalongkorn University, Bangkok, Thailand.
4 Laboratory of biotechnology, Chulabhorn Research Institute, Bangkok, Thailand.
5 Undergraduate school, DDS Program, Faculty of Dentistry, Chulalongkorn University, Bangkok, Thailand.
6 Oral Biology Research Center, Faculty of Dentistry, Chulalongkorn University, Bangkok, Thailand.
7 Department of Oral Pathology, Faculty of Dentistry, Chulalongkorn University, Bangkok, Thailand.
Corresponding author: Supreda Suphanantachat Srithanyarat E-mail: supreda.s@chula.ac.th,
Oranart Matangkasombut E-mail: oranart.m@chula.ac.th
Total Article Views
Editor: Anak Iamaroon,
Chiang Mai University, Thailand
Article history:
Received: February 8, 2023;
Revised: May 9, 2023;
Accepted: June 21, 2023;
Published online, June 30, 2023

