
Gonad Homogeneity, Maturity, and Gonado-Somatic Index of Wild Indonesian Eel (Anguilla bicolor bicolor McClelland, 1844)
Bambang Retnoaji*, Adhi Prasetyo, Nur Indah Septriani, Fajar Sofyantoro, and Noritaka MochiokaPublished Date : July 10, 2023
DOI : https://doi.org/10.12982/NLSC.2023.046
Journal Issues : Number 3, July-September 2023
Abstract The current study was conducted to investigate the homogeneity of gonad maturity in Anguilla bicolor bicolor, and whether Gonado-Somatic Index (GSI) is a reliable indicator of this maturity. Eel samples were obtained from fishermen at Segara Anakan, Cilacap, Central Java, Indonesia in December 2015, and May 2017. Fourteen specimens were analyzed based on their gonad histological structure from cranial, medial, and caudal portions and were measured for GSI values. The present study shows that there is a synchronization of oocyte maturity time on the medial, cranial and caudal parts. It might suggest that during the maturation process, Anguilla develop into silver eels and return to the sea to spawn. The highest GSI value is 2.67 in the female eel containing the highest number of mid-vitellogenic oocytes. Based on these results, it can be suggested that GSI are a suitable parameter for measuring the gonadal maturity of female A. bicolor bicolor.
Keywords: Gonadal maturity, Homogeneity, Indonesian eel, A. bicolor bicolor, Segara Anakan
Funding: We are grateful to The Ministry of Education, Science and Culture in Japan (MEXT) and Faculty of Biology, Universitas Gadjah Mada for the funding.
Citation: Retnoaji, B., Prasetyo, A., Septriani, N. I., Sofyantoro, F., and Mochioka, N. 2023. Gonad homogeneity, maturity, and gonado-somatic index of wild indonesian eel (Anguilla bicolor bicolor McClelland, 1844). Natural and Life Sciences Communications. 22(3): e2023046.
INTRODUCTION
There are seven species and subspecies of tropical eels from the genus Anguilla in Indonesian waters e.g. A. celebesensis, A. interioris, A. bengalensis bengalensis, A. marmorata, A. borneensis, A. bicolor bicolor, and A. bicolor pacifica (Sugeha et al. 2008). In Southeast Asian regions, A. bicolor bicolor has been found in Indonesia and Malaysia (Sugeha et al. 2008; Arai and Abdul Kadir 2017), Thailand (Tongnuinui et al 2016), the Philippines (Briones et al. 2007), Myanmar and Viet Nam (Suryati et al. 2019). A. bicolor bicolor population in nature has significantly decreased due to over-harvesting in recent years. Moreover, it is currently listed as ‘Near Threatened’ by the International Union for Conservation of Nature (IUCN) Red List of Threatened Species (Jacoby et al. 2014; Pike et al 2020). Therefore, studies about reproductive characteristics of this species are important for monitoring and conservatory purposes.
Reproductive characteristics of A. bicolor bicolor, including the spawning time, the gonad development, and maturity has been previously reported (Arai et al. 2016; Arai and Abdul Kadir 2017). The spawning of female A. bicolor bicolor in Segara Anakan, Indonesia occurs throughout the year, in contrast with temperate eels that typically spawn in autumn (Arai et al. 2016). As the final preparation of spawning, the mid-vitellogenic oocytes develop and the Gonado-Somatic Index (GSI) reaches 4.5 (Arai et al. 2016). The GSI of A. bicolor bicolor increases along with the advancing of its maturation stage (Arai et al. 2016; Arai and Abdul Kadir 2017). Meanwhile, male A. bicolor bicolor collected from Penang, Malaysia only showed the spermatogonia stage, suggesting that the male eels might be in the premature stages before the migration starts (Arai and Abdul Kadir 2017).
Gonad homogeneity is a term that refers to the consistency of gonadal development within a single individual (Tricas and Hiramoto, 1989). This concept is particularly important in the study of female gonads, as it helps to identify the synchronization of oocyte maturation, which is essential for the success of reproduction. The level of homogeneity of the gonads may vary between different individuals and even within the same individual over time (Tricas and Hiramoto, 1989). Therefore, assessing gonad homogeneity is a crucial step in understanding the reproductive biology of eels, particularly in the context of reproductive physiology and endocrinology studies. Previous studies of gonad development homogeneity in temperate eels showed that the left gonad is heavier, longer, and contains more eggs compared to the right one (Matsui 1957; Todd 1981; Tesch 2003). In contrast with the right gonad that extends towards the anterior, the left gonad exhibits posterior extension (Todd 1981; Tesch 2003). However, study on the gonad histology structure, including homogeneity, in tropical eels is lacking. Therefore, the current study aimed to elucidate the homogeneity of gonadal maturation in A. bicolor bicolor and determine the suitability of GSI as a metric for assessing gonadal maturity.
MATERIALS AND METHODS
Fish collection and morphometric measurement
Eel samples were obtained from Segara Anakan, Cilacap, Central Java, Indonesia in December 2015, and May 2017 during wet and dry season, respectively(Prasetyo, 2017). The physiochemical parameters that were measured from August 2015 to January 2016 showed that the depth ranged from 3.74 ± 2.22 to 5.58 ± 4.06 meters, the total suspended solid ranged from 9.40 ± 1.67 to 23.80 ± 12.07, the temperature ranged from 27.44 ± 0.52 to 31.32 ± 0.22 °C, the pH was recorded at 7.00 ± 0.00, and the salinity ranged from 26.60 ± 4.28 to 35.40 ± 1.82 (Pratiwi et al., 2018). In total, 14 eels were transported to the Laboratory of Animal Structure and Development, Faculty of Biology, Universitas Gadjah Mada, and stored in an aquarium prior to dissection (Figure 1). Since the IUCN categorizes A. bicolor bicolor as "Near Threatened" species, collecting a large number of samples from the wild are prohibited. Therefore, a sample size of 14 is deemed to be adequate, considering that 3-5 samples of each developmental stage were obtained as representatives. Morphometric parameters include measuring the total length and body weight. Total length was measured using a digital caliper and measuring tape. Body weight was measured using semi analytical scales. Species identification based on morphological observations showed that the anodorsal length ranges between -1.08 to 3.43 cm, indicating that the eels belong to the short-finned eels as reported by Robinet and Feunteun (2002). Meanwhile, based on the geographical distribution of the short-finned eel in the South Java Sea, the eels were categorized as A. bicolor bicolor (Sugeha et al. 2008).

Figure 1. Representative samples of (A) male and (B) female A. bicolor bicolor obtained from Segara Anakan, Cilacap, Central Java, Indonesia.
Histological staining of gonads
The gonads were prepared using paraffin method for microscopic observations. Gonads were fixed with Bouin’s solution for 3 hours, followed by dissection according to the standard protocol and stained with Hematoxylin Eosin (HE) or Mallory Acid Fuchsin (MAF) staining (Bancroft et al. 1994). The slides were microscopically identified for oocyte development observation.
Gonado-Somatic Index (GSI), Eye Index (EI), and Pectoral Fin Index (PFI)
The GSI was calculated as follows:
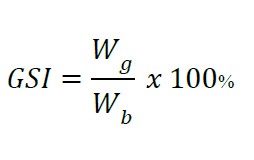
Wg: gonad weight
Wb: total body weight
The EI was calculated as follows:
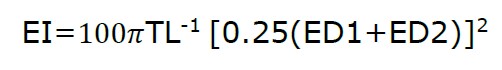
ED1: eye horizontal diameter
ED2: eye vertical diameter
The PFI was calculated as follows:
PFI = 100 PFL TL-1
PFL: Pectoral Fin Length
Data analysis
Data analyses were conducted by qualitative and quantitative methods. SPSS software ver.16 was used for Bivariate correlation statistical analysis of body length, body weight, gonad weight, and GSI. Kruskal Wallis test continued with Mann Whitney U-test was performed to examine differences in the percentage of oocyte development between cranial, medial, and caudal gonads of female eels.
RESULTS
The morphometry and GSI values of eels collected from Segara Anakan are shown in Table 1. Eel samples with the highest value of total length, total weight, gonad weight, and GSI is G. Among the 14 samples obtained from Segara Anakan, we identified yellow females (n=3), silver female (n=4), undifferentiated (n=3), and silver male (n=4).
Table 1. Morphometry, Gonado-Somatic Index (GSI), Eye Index (EI), and Pectoral Fin Index (PFI) of male, female, and undifferentiated A. bicolor bicolor.
|
Sample |
Total length (cm) |
Total weight (g) |
Gonad weight (g) |
GSI |
EI |
PFI |
Stage and sex |
|
A |
33.00 |
45.00 |
0.11 |
0.24 |
2.72 |
2.91 |
yellow female |
|
B |
39.50 |
80.00 |
0.28 |
0.35 |
3.32 |
3.02 |
yellow female |
|
C |
55.00 |
220.00 |
1.63 |
0.74 |
5.06 |
3.83 |
yellow female |
|
D |
60.00 |
370.00 |
4.82 |
1.3 |
12.23 |
5.30 |
silver female |
|
E |
61.00 |
380.00 |
4.48 |
1.18 |
6.85 |
5.98 |
silver female |
|
F |
61.00 |
330.00 |
5.24 |
1.59 |
13.45 |
5.08 |
silver female |
|
G |
78.50 |
1,200.00 |
32.08 |
2.67 |
10.21 |
4.83 |
silver female |
|
H |
27.00 |
20.00 |
0.05 |
0.25 |
1.46 |
2.7 |
undifferentiated |
|
I |
38.00 |
90.00 |
0.68 |
0.76 |
2.76 |
3.08 |
undifferentiated |
|
J |
40.50 |
70.00 |
0.82 |
1.17 |
3.93 |
3.22 |
undifferentiated |
|
K |
41.90 |
131.76 |
2.13 |
1.62 |
10.49 |
5.07 |
silver male |
|
L |
44.80 |
143.50 |
1.22 |
0.85 |
12.85 |
5.21 |
silver male |
|
M |
46.70 |
182.15 |
1.73 |
0.95 |
8.74 |
5.06 |
silver male |
|
N |
46.80 |
169.64 |
1.37 |
0.81 |
12.52 |
5.81 |
silver male |
Based on the simple correlation test (Bivariate correlation), body length, body weight, and gonad weight showed significant correlation with GSI values (Table 2).
Table 2. Pearson correlation values of Body Weight (BW), Gonad Weight (GW), Total Length (TL), and Gonado-Somatic Index (GSI) of eel samples.
|
|
TL |
BW |
GW |
GSI |
|
TL |
1 |
0.88 |
0.77 |
0.82 |
|
BW |
0.88 |
1 |
0.98 |
0.84 |
|
GW |
0.77 |
0.98 |
1 |
0.82 |
|
GSI |
0.82 |
0.84 |
0.82 |
1 |
The histological structure of A. bicolor bicolor gonads was shown in Figure 2. Figure 2H–J show undifferentiated gonads of Primordial Germ Cells (PGC) which could not be distinguished as male or female gonad cells. Undifferentiated gonads were found in eels with the following measurements: GSI values of 0.25–1.17, body weight of 20–90 g, and body lengths of 27–40.5 cm. Meanwhile, Figure 2A–G displays gonads that underwent differentiation into female gonads of different stages.
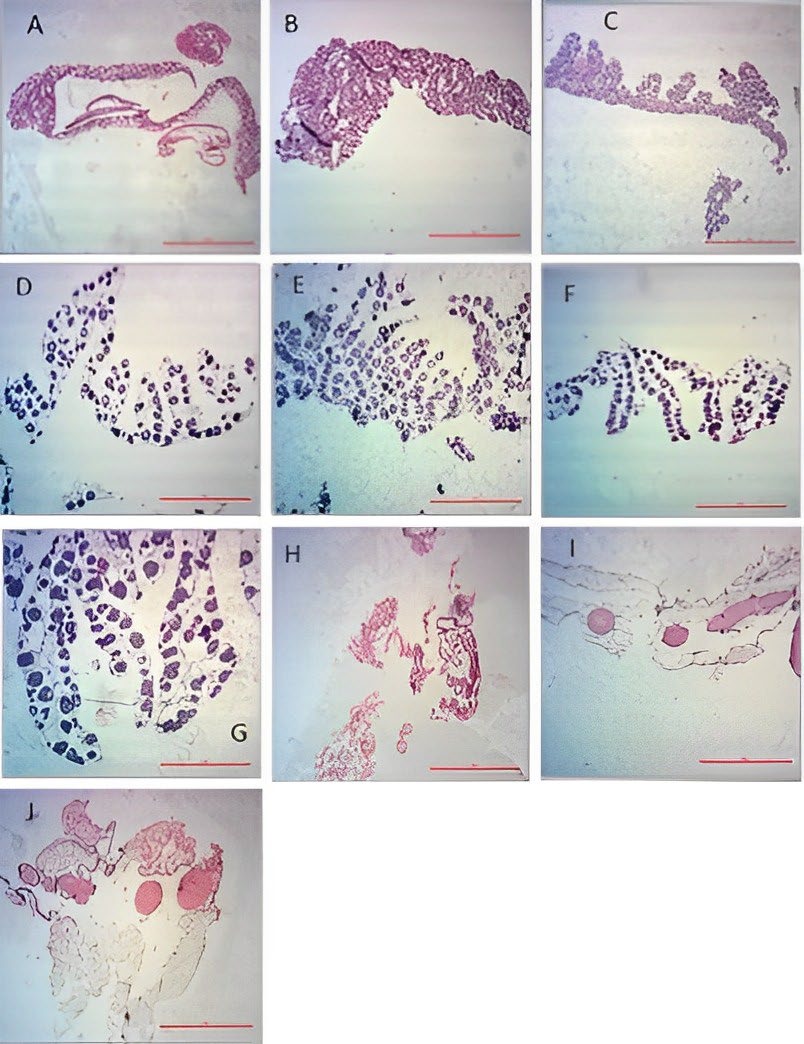
Figure 2. Photomicrographs sections of ovaries of female (A-G) and undifferentiated (H-J) A. bicolor bicolor samples. (Hematoxylin-Eosin staining; 40x magnification; 100 µm scale).
Development of female ovaries of A. bicolor bicolor are indicated by oogonia, primary oocyte, cortical alveoli, vitellogenic, and mid-vitellogenic as shown in Figure 3. In the yellow eels, the gonads were dominated by primary oocytes with few visible oogonia. Oocytes with chromatin nucleolus or perinuclear surrounded with basophilic cytoplasm, few oil droplets, and nucleolus were observed. In the silver eel, vitellogenic oocytes contained a relatively higher number of oil droplets in cytoplasm. Although the cytoplasm is strongly basophilic, it contains peripheral yolk granules accumulating in the peripheral region of oocytes, and smaller nucleolus located in the peripheral nucleus. Mid-vitellogenic and oocytes containing peripheral yolk granules and central yolk platelets were noticeable. Furthermore, in this stage, the nucleus size was relatively bigger with disappearing nucleolus, increased number and size of oil droplets and cortical alveoli, and thickening chorion.
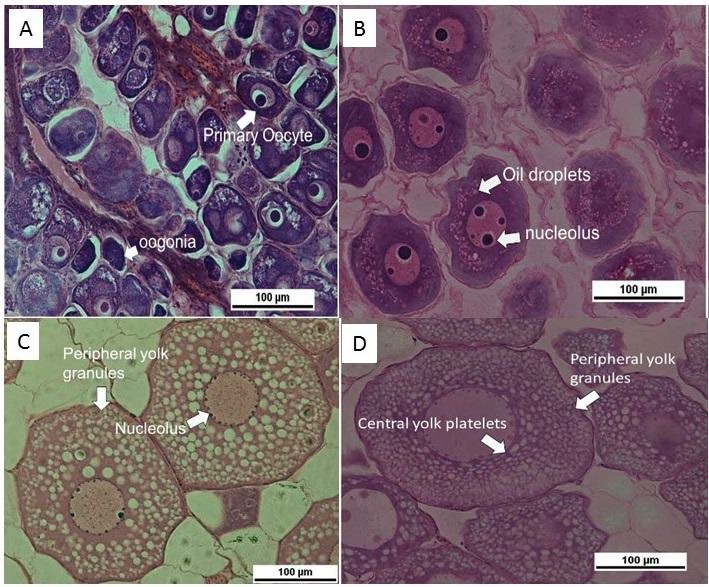
Figure 3. Oocyte development of female A. bicolor bicolor (Hematoxylin-eosin staining, 400x magnification, 100µm scale). A. Oogonia and primary oocyte; B. Cortical alveoli; C. vitellogenic; D. Mid-vitellogenic.
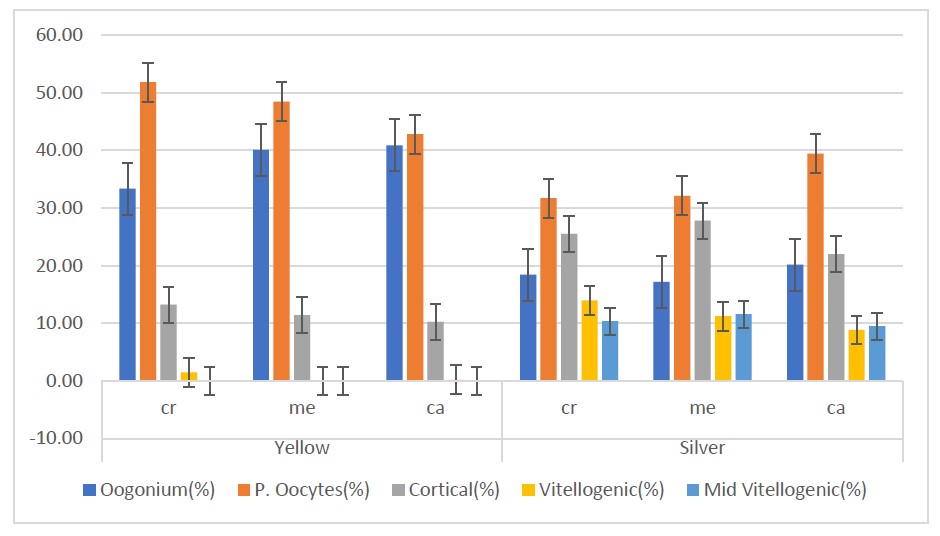
Figure 4. Percentage of oocytes development in yellow and silver eel samples. Cr: cranial; Me: median; Ca: caudal.
As shown in Figure 4, in both yellow and silver eels, the results of the Kruskal Wallis test indicate that the distribution of oocytes in the cranial, medial, and caudal regions are not significantly different. However, there is a significant difference in the distribution of vitellogenic stage between the two stages. The Mann Whitney U-test revealed that the caudal gonad of silver eels had a significantly higher number of vitellogenic oocytes compared to the medial and caudal gonads of yellow eels. Additionally, the number of vitellogenic oocytes in the cranial part of silver eels was significantly higher than that of yellow eels.
The development of oocytes in the cranial, caudal, and medial tends to be homogeneous in the same stage. These results suggest that most of the oocytes in the cranial, medial, and caudal gonads were in the same phase of development. The results of the analysis for gonadal development showed that the number of vitellogenic significantly increased during yellow changes to silver eel.
Male eels can be identified by the testicles that were lobed and oval surrounded by adipose tissue (Figure 5). The testicles were covered and protected by thick dense connective tissue on the outside (capsule) and were composed of spermatogenic cells consisting of spermatogonia with the characteristics of having a bright cytoplasm, large and round cell nuclei, and round nucleoli. The spermatogonia cells surround the basal portion of the seminiferous tubules. The cytoplasm of the spermatogenic cells and the Sertoli cells was shown in red. Meanwhile, the interstitial tissue of the testes, located between the seminiferous tubules, consists of connective tissue, Leydig cells, smooth muscle, and blood vessels. Connective tissue consists of blue-stained collagen fibers and red-stained smooth muscle/blood vessels, both of which could not be distinguished on histological preparations. The Leydig cells, located between the interstitial connective tissue fibers, were highlighted in red. Additionally, spermatozoa with flagella were identified in the lumen of seminiferous tubule.
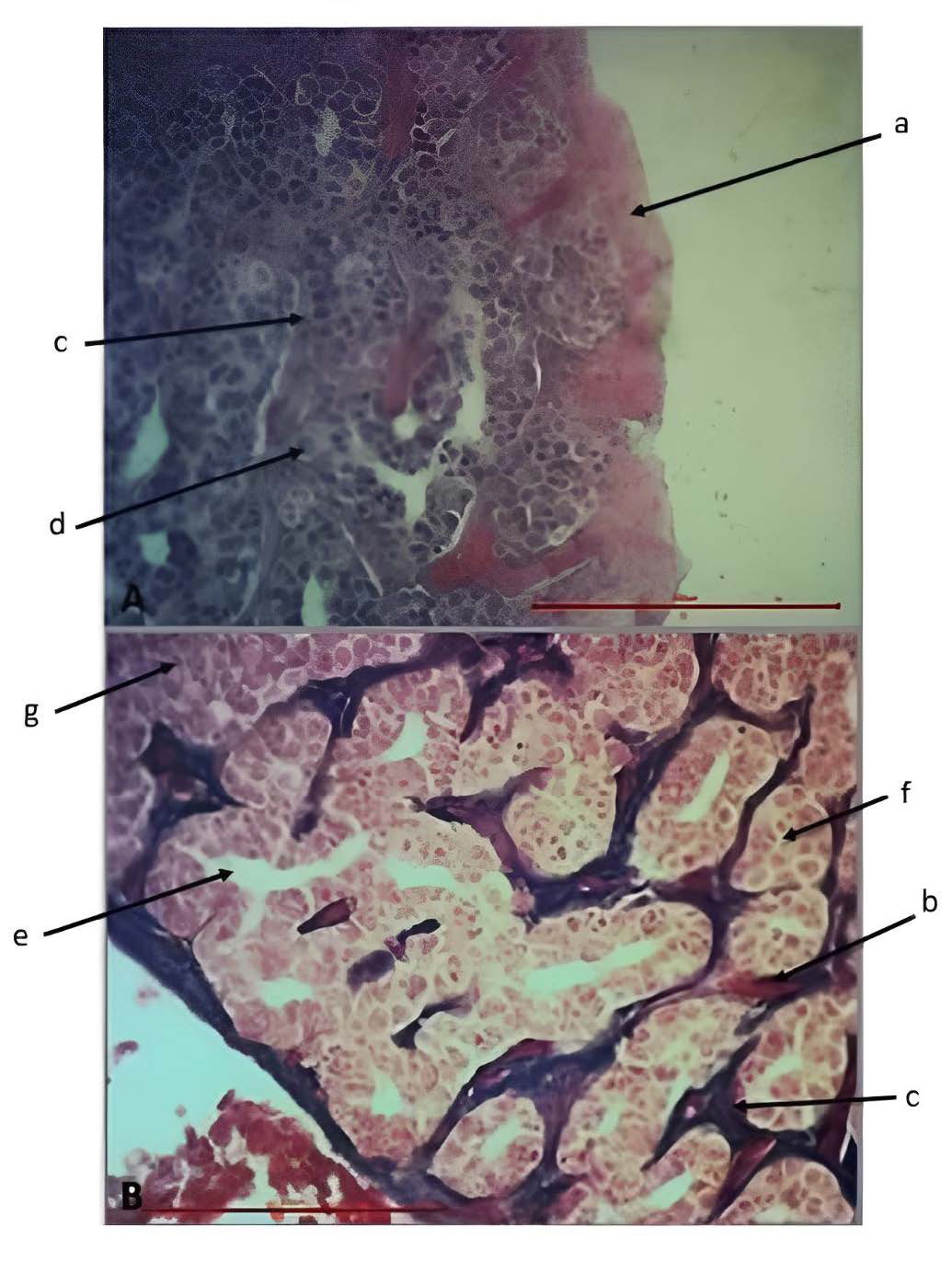
Figure 5. Histology of male A. bicolor bicolor gonads with (A) Hematoxylin-eosin (HE) and (B) Mallory Acid Fuchsin (MAF) staining (400x magnification; 100µm scale). a= dense connective tissue (capsule), b = blood vessels or smooth muscle, c = collagen connective tissue, d = Sertoli cells, e = lumen of seminiferous tubules, f = seminiferous tubules, g = Leydig cells.
DISCUSSION
The current study documented the gonad homogeneity in female A. bicolor bicolor by observing the development of gonad maturity and index on the cranial, medial, and caudal area. In addition, our data exhibit the histological structure of the gonads in male A. bicolor bicolor found in Segara Anakan, Indonesia. Our findings also reveal that the gonad maturity of female A. bicolor bicolor is homogeneous, with oocytes in the cranial, medial, and caudal showing homogeneity in the time of maturity. It is supported by the evidence that Anguilla undergoes maturation and transform into silver eels which migrate to the sea for spawning and eventually die (Podda et al. 2020; Aprahamian et al. 2021; Jellyman 2022; Piper et al. 2022).
Studies on the development of gonad maturity have been carried out at the developmental stage of A. anguilla (Colombo et al. 1984; Colombo and Grandidr 1996; Palstra et al. 2007; Palstra et al. 2011; Geffroy and Bardonnet 2016). Additionally, similar studies of A. japonica (Han et al. 2003; Gong et al. 2017), A. australlis and A. diefenbachii (Todd 1981; Lokman et al. 1998), A. reinhardtii (Walsh et al. 2003), A. bicolor bicolor and A. bengalensis bengalensis (Robinet et al. 2003; Arai et al. 2016; Tongnunui et al. 2016; Arai and Abdul Kadir 2017), and A. rostrata have been reported (Wenner and Musick 1974). Previous studies revealed that the histological development of gonads in female eels can be described by observing the oogonia, primary oocytes, cortical alveoli, and vitellogenic, which indicate the availability of the immature stage, advanced immature stage, and mature stage of the gonads. Before migrating to the spawning site, previous studies reported that the silver stage was observed in the vitellogenic of temperate eels (Lokman et al. 1998; Han et al. 2003; Palstra et al. 2007), and in the mid-vitellogenic (Arai et al. 2016; Arai and Abdul Kadir 2017) or late vitellogenic of tropical eels (Tongnunui et al. 2016). Our findings indicate that in female eels transitioning from yellow to silver stage, the oogonium, primary oocytes, and cortical alveoli develop into vitellogenic and mid-vitellogenic stages. It might indicate that the distance of spawning sites to coastal water is closer in tropical eels compared to temperate eels.
In the form of germs cells, the glass eel gonads could not be distinguished clearly. After sex differentiation occurs, the oogonia turn into oocytes and initiate the meiotic division (Yamamoto and Yamauchi 1974). Oocytes then begin the initial division of prophase and are arrested at the diplotene stage (Yamamoto and Yamauchi 1974). Oocytes in the primary growth stage begin to appear at the yellow stage, upon reaching a total length of 20-30 cm. The primary growth stages are differentiated into the nucleolus and the perinucleolar stage (Yamamoto and Yamauchi 1974). The secondary growth is characterized by the presence of oil droplets found in the cytoplasm, which then increases in number and size along with the silverization stage (Yamamoto and Yamauchi 1974).
In cultivated silver eels injected with Salmon Pituitary Homogenate (SPH), the oocytes develop at the secondary yolk stages and mid-vitellogenic, in which the oil droplets and cortical alveoli increase in number and size followed by thickening of the chorion (Palstra et al. 2005; Ijiri et al. 2011; Lokman et al. 2015). Afterwards, the late stages are initiated and characterized by an increase in oocyte diameter (850-900 um) due to hydration and germinal vesicle migration (Adachi et al. 2003).
The sex of eels can be easily determined visually when they are over 30 cm in total length for other anguillid eel species (Melia et al. 2006). However, three individuals with the total length of 40 cm were "undifferentiated" in this study. This discrepancy might be due to individual variation. Our result is also consistent with previous reports stating that GSI values can be used to reflect the development of gonads in eel (Durif et al. 2006).
Secondary growth of oocytes (vitellogenic stage) was observed in eels with a length of 55 cm, indicating that female A. bicolor bicolor in Segara Anakan reach the important phase of reproduction at that length. Therefore, this data may serve as a reference for fishermen not to catch eels with a total length of 55 cm or more. In contrast to previous studies in A. anguilla (Walsh et al. 2003) and A. australis (Todd 1981) that measured the diameter of the oocytes, the current study calculated the size of oocytes by measuring the circumference. The biggest circumference was found in the mid-vitellogenic phase of the female eel G (144.01 ± 27.59 µm). The result is consistent with previous findings reporting that that mid-vitellogenic phase is larger than the other phases, because it contains cortical alveoli causing the enlargement of cytoplasm (Adachi et al. 2003).
Our study is the first to prove the existence of male A. bicolor bicolor in Segara Anakan, compared to the previous studies that only found female A. bicolor bicolor (Arai et al. 2016). In male eels examined in the current study, spermatogonia dominates the seminiferous tubules, indicating that the testes may not have experienced gonadal maturity yet, even upon reaching the silver stage. Similar results have also been reported in male A. bicolor bicolor and A. bengalensis bengalensis in Penang, Malaysia showing spermatogonia phase (Arai and Abdul Kadir 2017). Therefore, it might be suggested that the gonads of male tropical eels ripen during migration to the spawning area. Rahmadya et al. suggested that the morphological observations need to be supported with ecological parameters to present a comprehensive study of eels in Indonesia (Rahmadya et al. 2022). Therefore, further observations related to the habitat A. bicolor bicolor needs to be explored for future research. It is also of interest to compare the gonadal maturity of eels found in freshwater and brackish water areas in Indonesia.
CONCLUSION
The current study demonstrates the homogeneity of oocyte maturation across the medial, cranial, and caudal sections in A. bicolor bicolor. The female eel with the highest number of mid-vitellogenic oocytes had the highest GSI value of 2.67, indicating that GSI is a suitable metric for assessing the gonadal maturity of female A. bicolor bicolor.
ACKNOWLEDGMENTS
The authors would like to thank A. Hananya, C. Ratucoreh, J. Saraswati, G. Kerans, and R. Trianasari for assisting in data collection.
AUTHOR CONTRIBUTIONS
Noritaka Mochioka (NM) and Bambang Retnoaji (BR) designed the research. Adhi Prasetyo (AP) and Nur Indah Septriani (NIS) performed the experiments and collected the data. AP, NIS, FS, NM, and BR analyzed the data and wrote the manuscript. NM and BR supervised all the processes.
ETHICAL STATEMENT
The experimental design was approved by Laboratory of Integrated Research and Testing (LPPT), Universitas Gadjah Mada, Indonesia (No. 00117/04/LPPT/II/2017).
CONFLICT OF INTEREST
The authors declare that they hold no competing interests.
REFERENCES
Adachi, S., Ijiri, S., Kazeto, Y., and Yamauchi, K. 2003. Oogenesis in the Japanese eel, Anguilla japonica. p. 301-331. In: Aida, K., Tsukamoto, K., Yamauchi, K. [eds.], Eel Biology. Springer, Tokyo.
Aprahamian, M.W., Evans, D.W., Briand, C., Walker, A.M., McElarney, Y., and Allen, M. 2021. The changing times of Europe’s largest remaining commercially harvested population of eel Anguilla anguilla L. Journal of Fish Biology. 99: 1201–1221.
Arai, T., and Abdul Kadir, S.R. 2017. Opportunistic spawning of tropical anguillid eels Anguilla bicolor bicolor and A. bengalensis bengalensis. Scientific Reports. 7: 1-17.
Arai, T., Abdul Kadir, S.R., and Chino, N. 2016. Year-round spawning by a tropical catadromous eel Anguilla bicolor bicolor. Marine Biology. 163: 1-7.
Bancroft, J.D., Cook, H.C., and Stirling, R.W. 1994. Manual of histological techniques and their diagnostic application, in: Manual of histological techniques and their diagnostic application. 457– 457.
Briones, A.A., Yambot, A.V., Shiao, J-C., Iizuka, Y., and Tzeng, W-N. 2007. Migratory pattern and habitat use of tropical eels Anguilla spp. (Teleostei: Anguilliformes: Anguillidae) in the Philippines, as revealed by otolith microchemistry. The Raffles Bulletin of Zoology. 14: 141-149.
Colombo, G., Grandi, G., and Rossi, R. 1984. Gonad differentiation and body growth in Anguilla anguilla L. Journal of Fish Biology. 24: 215–228.
Colombo, G., and Grandidr, G. 1996. Histological study of the development and sex differentiation of the gonad in the European eel. Journal of Fish Biology. 48: 493–512.
Durif, C., Dufour, S., and Elie, P. 2006. Impact of silvering stage, age, body size and condition on reproductive potential of the European eel. Marine Ecology Progress Series. 327: 171–181.
Geffroy, B., and Bardonnet, A. 2016. Sex differentiation and sex determination in eels: consequences for management. Fish and Fisheries. 17: 375–398.
Gong, X., Wang, D., Bao, B., Zhang, Q., Jiang, X., and Liu, L. 2017. Gonadal development and silvering of the Japanese Eel (Anguilla japonica) in the Yangtze River during downstream migration. Aquaculture and Fisheries. 2: 173–178.
Han, Y-S., Liao, I-C., Huang, Y-S., He, J-T., Chang, C-W., and Tzeng, W-N. 2003. Synchronous changes of morphology and gonadal development of silvering Japanese eel Anguilla japonica. Aquaculture. 219: 783–796.
Ijiri, S., Tsukamoto, K., Chow, S., Kurogi, H., Adachi, S., and Tanaka, H. 2011. Controlled reproduction in the Japanese eel (Anguilla japonica), past and present. Aquaculture Europe. 36: 13–17.
Jacoby, D.M.P., Harrison, I.J., and Gollock, M.J. 2014. Anguilla bicolor. In: Anguilla bicolor. The IUCN red list of threatened species 2014: e. T166894A6701570.
Jellyman, D.J. 2022. An enigma: How can freshwater eels (Anguilla spp.) be such a successful genus yet be universally threatened? Reviews in Fish Biology and Fisheries. 32: 701–718.
Lokman, P.M., Vermeulen, G.J., Lambert, J.G.D., and Young, G. 1998. Gonad histology and plasma steroid profiles in wild New Zealand freshwater eels (Anguilla dieffenbachii and A. australis) before and at the onset of the natural spawning migration. I. Females. Fish Physiology and Biochemistry. 19: 325–338.
Lokman, P.M., Wylie, M.J., Downes, M., Di Biase, A., and Damsteegt, E.L. 2015. Artificial induction of maturation in female silver eels, Anguilla australis: The benefits of androgen pre-treatment. Aquaculture. 437: 111–119.
Matsui, I. 1957. On the record of a leptocephalus and catadromous eels of Anguilla japonica in the waters around Japan with a presumption of their spawning places. Journal of Shimonoseki College of Fisheries. 7: 151–167.
Melia, P., Bevacqua, D., Crivelli, A.J., De Leo, G.A., Panfili, J., and Gatto, M. 2006. Age and growth of Anguilla anguilla in the Camargue lagoons. Journal of Fish Biology. 68: 876–890.
Palstra, A., Curiel, D., Fekkes, M., de Bakker, M., Székely, C., van Ginneken, V., and van den Thillart, G. 2007. Swimming stimulates oocyte development in European eel. Aquaculture. 270: 321–332.
Palstra, A.P., Cohen, E.G.H., Niemantsverdriet, P.R.W., van Ginneken, V.J.T., and van den Thillart, G.E.E.J.M. 2005. Artificial maturation and reproduction of European silver eel: Development of oocytes during final maturation. Aquaculture. 249: 533–547.
Palstra, A.P., Guerrero, M.A., de Laak, G., Klein Breteler, J.P.G., and van den Thillart, G.E.E.J.M. 2011. Temporal progression in migratory status and sexual maturation in European silver eels during downstream migration. Fish Physiology and Biochemistry. 37: 285–296.
Pike, C., Crook, V., Jacoby, D. and Gollock, M. 2020. Anguilla bicolor (amended version of 2019 assessment). The IUCN Red List of Threatened Species 2020: e.T166894A176494582.
Piper, A., Belen, A., Villanueva, B., and Gollock, M. 2022. Residence, activity patterns and behaviour of the giant mottled eel Anguilla marmorata in two freshwater protected areas in the Philippines. Aquatic Conservation: Marine and Freshwater Ecosystem. 32: 1606-1617.
Podda, C., Palmas, F., Frau, G., Chessa, G., Culurgioni, J., Diciotti, R., Fois, N., and Sabatini, A. 2020. Environmental influences on the recruitment dynamics of juvenile European eels, Anguilla anguilla, in a small estuary of the Tyrrhenian Sea, Sardinia, Italy. Aquatic Conservation: Marine and Freshwater Ecosystems. 30: 1638–1648.
Prasetyo, A. 2017. Histological studies and Gonadosomatic Index of gonadal development of eel (Anguilla bicolor bicolor McClelland, 1844). Thesis. Faculty of Biology, Universitas Gadjah Mada, Yogyakarta, Indonesia.
Pratiwi, H., Damar, A., and Sulistiono, S. 2018. Phytoplankton community structure in the estuary of Donan River, Cilacap, Central Java, Indonesia. Biodiversitas Journal of Biological Diversity. 19: 2104-2110.
Rahmadya, A., Ridwansyah, I., Daruati, D., Rahmat, A., Triyanto, T., Dewi, A.P., and Wildan, D.M. 2022. A systematic literature review of the methodology preference for analyzing eel (Anguilla spp.) habitat. Chiang Mai University Journal of Natural Sciences. 21:1-11.
Robinet, T., and Feunteun, E. 2002. First observations of shortfinned Anguilla bicolor bicolor and longfinned Anguilla marmorata silver eels in the Réunion Island. Bulletin Français de la Pêche et de la Pisciculture. 364: 87-95.
Robinet, T., Sbaihi, M., Guyet, S., Mounaix, B., Dufour, S., and Feunteun, E. 2003. Advanced sexual maturation before marine migration of Anguilla bicolor bicolor and Anguilla marmorata at Réunion Island: Sexual maturation of Anguilla. Journal of Fish Biology. 63: 538–542.
Sugeha, H.Y., Suharti, S.R., Wouthuyzen, S., and Sumadhiharga, K. 2008. Biodiversity, distribution, and abundance of the tropical Anguillid eels in the Indonesian waters. Marine Research in Indonesia. 33: 129–137.
Suryati, N. K., Pamungkas, Y. P., and Muthmainnah, D. 2019. Addressing the issues and concerns on Anguillid eel fisheries in Southeast Asia. Fish for the People. 17: 19-24.
Tesch, F-W. 2003. The Eel, 3rd edn. Ed. by J. E. Thorpe. Oxford: Blackwell Science.
Tricas, T. C., and Hiramoto, J. T. 1989. Sexual differentiation, gonad development, and spawning seasonality of the Hawaiian butterflyfish, Chaetodon multicinctus. Environmental Biology of Fishes. 25: 111-124.
Todd, P.R. 1981. Morphometric changes, gonad histology, and fecundity estimates in migrating New Zealand freshwater eels (Anguilla spp.). New Zealand Journal of Marine and Freshwater Research. 15: 155–170.
Tongnunui, P., Yoknoi, N., Pechnoi, P., Yamada, H., and Kon, K. 2016. The first record of female maturation of the short-finned eel, Anguilla bicolor bicolor, in the coastal waters of Thailand. Tropical Life Sciences Research. 27: 145–152.
Walsh, C.T., Pease, B.C., and Booth, D.J. 2003. Sexual dimorphism and gonadal development of the Australian longfinned river eel: Gonadal development in Australian river eel. Journal of Fish Biology. 63: 137–152.
Wenner, C.A., and Musick, J.A. 1974. Fecundity and gonad observations of the American eel, Anguilla rostrata, migrating from Chesapeake Bay, Virginia. Journal of the Fisheries Research Board of Canada. 31: 1387–1391.
Yamamoto, K., and Yamauchi, K. 1974. Sexual maturation of Japanese eel and production of eel larvae in the aquarium. Nature. 251:220–222.
OPEN access freely available online
Natural and Life Sciences Communications
Chiang Mai University, Thailand. https://cmuj.cmu.ac.th
Bambang Retnoaji1, *, Adhi Prasetyo1, Nur Indah Septriani1, 2, Fajar Sofyantoro1, and Noritaka Mochioka2
1 Department of Tropical Biology, Faculty of Biology, Universitas Gadjah Mada, Yogyakarta, Indonesia.
2 Faculty of Agriculture, Kyushu University, Fukuoka, Japan.
Corresponding author: Bambang Retnoaji E-mail: bambang.retnoaji@ugm.ac.id
Total Article Views
Editor: Veerasak Punyapornwithaya,
Chiang Mai University, Thailand
Article history:
Received: January 2, 2023;
Revised: May 12, 2023;
Accepted: May 16, 2023;
Published online: May 29, 2023

