
Preservation of Peripheral Blood Stem Cells (CD34+/CD38-) for Bone Marrow Transplantation in Thai Lymphoma Patients
Songyot Anuchapreeda*, †, Banphot Shaengkhamnang†, Pawaret Panyajai, Sawitree Chiampanichayakul, Singkome Tima, Phennapha Klangsinsirikul, Preeyanat Vongchan and Adisak Tantiworawit*Published Date : July 10, 2023
DOI : https://doi.org/10.12982/NLSC.2023.044
Journal Issues : Number 3, July-September 2023
Abstract Hematopoietic stem cell transplantation (HSCT) is one method of lymphoma therapy. Peripheral blood stem cell (PBSC) separation, preservation, cell viability, and cell function before transplantation are important factors in the success rate of stem cell transplantation. This study aims to separate and estimate the efficiency of deep-freezing preservation in autologous PBSCs (CD34+/CD38-) from lymphoma patients undergoing stem cell transplantation. PBSCs were separated and collected by leukapheresis before being cryopreserved and stored in liquid nitrogen. The number of CD34+/CD45dim cells was investigated and compared with the subpopulation of CD34+/CD38- cells using conventional trypan blue exclusion method and 7-AAD before and after cryopreservation. Colony-forming units (CFUs) were determined to indirectly assess the viability and potency of the PBSCs. The result showed that CD34+/CD38– cells constituted 35.56% of total CD34+ cells and 0.05% of total nucleated cells (TNCs). After thawing, the number of CD34+/CD38- cells did not demonstrate significant differences compared with pre-storage. The percentage of CFU recovery was 94.74%. In this study, the storage process of deep-freezing cryopreservation demonstrated high-quality recovery of CD34+/CD38- cells from PBSCs for autologous hematopoietic stem cell transplantation in lymphoma patients. This result showed novel data about the preservation of CD34+/CD38- cells.
Keywords: Lymphoma, Autologous peripheral blood stem cell, Blood cryopreservation, Bone marrow transplantation
Funding: This research project was supported by the Fundamental Fund 2022, Chiang Mai University, Thailand and the Faculty of Associated Medical Sciences, Chiang Mai University, Chiang Mai, Thailand.
Citation: Anuchapreeda, S., Shaengkhamnang, B., Panyajai, P., Chiampanichayakul, S., Tima, S., Klangsinsirikul, P., Vongchan, P., and Tantiworawit, A. 2023. Preservation of peripheral blood stem cells (CD34+/CD38-) for bone marrow transplantation in Thai lymphoma patients. Natural and Life Sciences Communications. 22(3): e2023044.
INTRODUCTION
Lymphoma is a lymphatic system malignancy leading to abnormal lymphocytic cell proliferation. In Thailand, in 2018, there were 192 cases of Hodgkin's lymphoma in males. There were 2,855 cases of non-Hodgkin's lymphoma in males (6.6 cases per 100,000 people in the population) and 2,487 cases in females (5.2 in 100,000). The incidence of non-Hodgkin's lymphoma (NHL) is higher than Hodgkin's lymphoma, encompassing 90% of total lymphoma cases (Suwanrungruang et al., 2021). NHL is the sixth most common malignancy in Thailand.
Advanced treatments have been recently developed, such as hematopoietic stem cell transplantation (HSCT). Routine HSCT treatments are autologous hematopoietic stem cell transplantation (autologous HSCT) and allogeneic hematopoietic stem cell transplantation (allogeneic HSCT). Autologous HSCT was developed to treat hematologic malignancies including lymphoma (Ali et al., 2015; Zahid et al., 2017) by destroying cancer and replacing the abnormal stem cells with normal patient’s hematopoietic stem cells (HSCs). Autologous HSC mobilization is conducted using chemotherapeutic treatments and/or combined with G-CSF to destroy the cancer cells and stimulate the release of HSCs from bone marrow into the bloodstream as peripheral blood stem cells (PBSCs). PBSCs are collected by leukapheresis and cryopreserved at the pretransplant step. Frozen PBSCs are thawed before reinfusion for transplantation. The assessment of PBSC quality depends on CD34+ cell concentration, dose, viability, function, and recovery. The recommended concentration of HSCs for successful engraftments is 2–5×106/kg (Sutherland et al., 1996; Rafiee et al., 2021). PBSC measurement by flow cytometry has complied with the ISHAGE, ISCT, and EBMT (Joint Accreditation Committee - ISCT & EBMT, 2021) guidelines including total nucleated cell (TNC) count, CD34+ cell count, cell viability, and percentage recovery.
CD34+/CD38- cells are human hematopoietic stem cells. They are functionally defined by their self-renewal capacity and multipotency, allowing the replenishment of normal blood cell types. Thus, CD34+/CD38- cells are important for stem cell transplantation in patients. PBSCs from lymphoma patients were separated and collected by leukapheresis machine. Measuring cell viabilities before and after storage is necessary since processing procedures and storage conditions highly affect CD34+ cell viability (Donmez et al., 2014; Fisher et al., 2014). Several studies found that cell viability and engraftment potential decreased after cryopreserved PBSC thawing (Castelhano et al., 2013; Berens et al., 2016; Facchin et al., 2022). Some studies have shown that PBSCs could be stored at 4°C for more than 48 h without significant loss of CD34+ cells and cell viability (Araújo et al., 2022). Therefore, PBSC preservation, cell viability, and cell function of CD34+/CD38- cells before transplantation are the main problems for HSCT.
This study investigated and compared the cell loss of CD34+/CD45dim and CD34+/CD38- subpopulations in PBSCs separated by leukapheresis before and after storage in deep-freezing cryopreservation. Cell viability was assessed via trypan blue exclusion (conventional method) and flow cytometry (7-AAD). The potency of CD34+ cells was assessed by CFU assay in a semi-solid medium to compare the number of total colony-forming unit and colony type (Akashi et al., 2000; Morgenstern et al., 2016; Pamphilon et al., 2013). These results were also compared to the clinical engraftment results. This study can be used as a guideline for CD34+/CD38- stem cell preservation, especially in lymphoma patients.
MATERIALS AND METHODS
Patients
Twenty-three collections of PBSCs were obtained from 10 patients (6 males and 4 females) with lymphoma who were receiving autologous-HSCT (Table 1). Normally, transplantation in lymphoma patients were 15–20 patients/year (data from the Stem Cell Transplantation Center, Department of Internal Medicine, Faculty of Medicine, Chiang Mai University, Thailand). All the lymphoma patients PBSC collections were performed from December 2017 to September 2018. This study was approved by the Ethics Committee of the Faculty of Medicine, Chiang Mai University, Chiang Mai, Thailand. The study code was NONE-2559-04138/Research ID: 4138; date of approval: September 23, 2016. PBSCs were assessed pre- and post-cryopreservation within 1–6 months after collection. Analysis of PBSCs included volume (mL), TNC count (×1010 cells), percentage recovery, CD34+ cell count (×106 cells/kg), CD34+/CD38- cell count (×106 cells/kg), and cell viability percentage. The potency of CD34+ cells was measured by CFU assay. The number of each colony type was counted (×104 cells) and calculated into percentage (%). PBSCs were compared and analysed in each collection bag before and after storage.
Table 1. Characteristics of autologous PBSCs (n = 23) from 10 autologous HSCT patients.
|
Parameter |
Male |
Female |
|
|
Age* (year) |
49.50 (29–59) |
||
|
Weight* (Kg) |
62.00 (44–80) |
||
|
Sex |
6 (60%) |
4 (40%) |
|
|
Total PBSCs (23 bags) Lymphoma (patients, bags) |
13 (56%) |
10 (44%) |
|
|
2 (7) 1 (1) 2 (4) 1 (1) |
3 (8) 0 1 (2) 0 |
|
Note: *Data presented as median (range)
PBSC separation, collection, aliquots, and thawing
Autologous PBSCs (23 blood collection bags) from lymphoma patients (n=10) were collected by leukapheresis (COMTEC, Fresenius, Waltham, MA, USA); target CD34+ yield in PBSCs was ≥ 2.0×106/kg of patient body weight. PBSC bags were centrifuged at 2,000×g, 8 min at 22°C (Sorvall RC3, Thermo Fisher Scientific, Waltham, MA, USA) to concentrate TNC and reduce plasma by approximately 20%. PBSC samples were immediately aliquoted from pre- and post-centrifugation of PBSCs into cryotubes (1 mL/tube) and measured for TNC and TNC recovery (%). The post-centrifuged PBSCs were aliquoted in two vials for pre-storage determination and CFU assay. Remaining post-centrifuged PBSCs were diluted with cryopreservative solution at the ratio of 1:1 in a cryobag. The cryopreservative solution was prepared by mixing DMSO and plasma with the final DMSO concentration adjusted to 20% and then stored at 4°C for 15 min. The final DMSO concentration after adding PBSCs was 10%. The cryopreserved PBSCs were aliquoted into two cryotubes (1 mL in each) for post-thawing determination and CFU assay. Then, cryopreserved PBSCs were pre-frozen at –20°C for 15 min and further deep-frozen in a controlled-rate freezer to –90°C. All the samples were stored in a liquid nitrogen tank (–180°C). Post-thawing samples were thawed at the indicated time period at 37°C in a water bath for 2–3 min. The samples were aliquoted into two tubes. The first tube was resuspended with EDTA at the sample to EDTA ratio of 500:50 for a viable TNC count using trypan blue exclusion and 7-AAD via flow cytometry analysis. The second tube was resuspended with heparin at a sample to heparin ratio of 500:80 for the CFU assay.
Interventional studies involving animals or humans, and other studies that require ethical approval, must list the authority that provided approval and the corresponding ethical approval code.
TNC, CD34+/CD45dim, and CD34+/CD38- cell counts
TNCs were counted using a cell counter (XE-5000, Sysmex, Kobe, Japan). HSC analysis was conducted using a modified ISHAGE protocol (Whitby et al., 2012). HSCs (CD34+/CD38–) were determined using stem cell enumeration kit (BD Biosciences, Franklin Lakes, NJ, USA). Briefly, the samples were diluted to 5×105 cells/100 µL with 0.5% BSA in PBS, pH 7.4. The sample (100 µL) was incubated with CD45-FITC/CD34-PE antibodies for CD34+ cells or HSC identification and anti-CD38 antibody (CD-38-PECy7) for identification of CD34+/CD38- subpopulation in a fluorescent bead tube (TruCount, BD Biosciences, Franklin Lakes, NJ, USA) followed by 20 min incubation at room temperature in the dark. After that, 2 mL red blood cell lysis (ammonium chloride, BD Biosciences, Franklin Lakes, NJ, USA) was added followed by incubation for 10 min in the dark and analysis within 1 h. HSCs were enumerated using a single platform analysis on a Cytomics FC500 flow cytometer (Beckman Coulter, Indianapolis, IN, USA) to identify target CD34+ cell (CD34+/CD45dim, SSlow) and subpopulation of HSCs (CD34+/CD38-).
Percentage of TNC recovery is calculated to evaluate the remaining nucleated cells after storage process which is one of good prognostic factors for survival rate in patients receiving transplantation. Percentage of TNC recovery was calculated using the following formula: (TNC post-storage/TNC pre-storage) × 100.
CD34+ enumeration was completed using a fluorescent bead tube collecting 75,000 CD45+ events/sample. The calculation formula was as follow:
(CD34+ or CD34+/CD38- cells)/µL = (number of CD34+ or CD34+/CD38- events/number of beads counted) × (total number of beads in each bead-coated tube) × (dilution factor/sample volume).
Cell viability test using trypan blue exclusion
The PBSCs were tested for their viability using trypan blue exclusion. The samples were diluted with PBS with a pH of 7.4 at a ratio of 1:10 and then mixed with an equal volume of trypan blue vital stain (0.4%) at room temperature for 5 min. The dead cells were identified by blue cytoplasm (stained cells as dead cells cannot exclude trypan blue dye). Stained and unstained cells were counted under 100x magnifying microscope (Olympus, Center Valley, PA, USA) and the percentage viability was calculated using the following formula: unstained cells/ (unstained cells + stained cells) × 100.
Cell viability test via flow cytometry
The samples were stained with 7-aminoactinomycin D (7-AAD) using stem cell enumeration kit (BD Biosciences, Franklin Lakes, NJ, USA) and analyzed with an FC500 flow cytometer (Beckman Coulter, Indianapolis, IN, USA). The nucleus was stained with 7-AAD to detect the viable cell signals by flow cytometry. This study measured the cell count and cell viability by diluting 5×105 cells/100 µL with 0.5% BSA in PBS, pH 7.4. BD-Trucount tube containing fluorescent beads was used to calculate the absolute cell count. The samples (100 µL) were incubated with 7-AAD (5 µL) in BD-Trucount tubes for 20 min at room temperature in the dark. Then 2 mL of lysing solution (1x ammonium chloride (NH4Cl) solution, BD Biosciences, Franklin Lakes, NJ, USA) was added into the tube, mixed by vortexing and incubated in the dark for 10 min. The samples were not fixed or washed before analysis. The sample tubes were placed in an ice bath and immediately analyzed within 1 h via flow cytometry (Cytomics FC500, Beckman, Coulter, Indianapolis, IN, USA). The results were analyzed using FlowJo analysis software based on ISHAGE guidelines.
CFU assay
Colony-forming units were used to indirectly assess the viability and potency of the PBSCs. The CFU assay represents the clonogenic potential of HSCs dividing to mature cells from viable progenitor cell lineages. Red blood cells in PBSCs were lysed by NH4Cl solution at the ratio of 1:4 followed by suspension and washing 3 times with 2% FBS, pH 7.4 in IMDM (InvitrogenTM, Carlsbad, CA, USA). A final cell suspension (1–5×104 cells) was resuspended in methylcellulose medium (HSC003, R&D Systems, Minneapolis, MN, USA) supplemented with cytokine cocktails (50 ng/mL SCF, 10 ng/mL GM-CSF, 10 ng/mL IL3, and 3 IU/mL EPO) and plated in a 35 mm dish in triplicate following the manufacturer’s instructions. The colony types from progenitors were identified and counted, including CFU-GEMM, CFU-GM, CFU-G, CFU-M, CFU-E, and BFU-E. The total colony count (×104 cells) and percentage CFU type were scored.
Percentage of CFU recovery is calculated to evaluate the proliferation and differentiation ability of individual cells within a sample after storage process which is one of good prognostic factors for survival rate in patients receiving transplantation. Percentage of CFU recovery was calculated using the following formula: (total CFU post-storage/ CFU pre-storage) × 100.
The percentage of cell loss is calculated to evaluate the amount of destroyed cells after storage that indicates the quality of storage process. The percentage of cell loss was calculated using the following formulation: (pre-storage cell count - post-storage cell count/pre-storage cell count) × 100.
Statistical analysis
All results were expressed as median (interquartile range, Q1–Q3) with a significant difference level of P < 0.05 or P < 0.001 and the differences were analyzed via the Mann-Whitney U test. Software for statistical analysis is IBM SPSS Statistics version 22 (Release 22.0.0.0).
RESULTS
Effects of deep-freezing in cryopreservation on CD34+, CD34+/38- cells, and TNC count assessed via flow cytometry
CD34+ cells were collected from the lymphoma patients, acquiring an average number of 0.45×106 (0.14–1.52×106) cells/kg. The CD34+ cell population in the TNCs after leukapheresis was 0.14%. The CD34+ cells lost after deep-freezing were 15.05% (9.73–25.58%). Pre-storage CD34+/CD38- cells were 0.16×106 (0.07–0.57×106) cells/kg. Thus, the CD34+/CD38- cell numbers were 35.56% of the total CD34+ cells and 0.05% of the TNCs. After thawing, CD34+/CD38- cells constituting 43.33% of the total CD34+ cells and 0.04% of the TNCs were not significantly different (P > 0.05) compared with those of pre-storage. Cell loss after deep-freezing was 30.17% (3.91–41.02%). After 1–6 months of cryopreservation, the number of CD34+ cells, CD34+/CD38- cells, and TNCs demonstrated no significant difference compared with the pre-storage (P > 0.05) data (Table 2).
Table 2. CD34+ stem cells, CD34+/CD38- cells, and TNC count from pre-storage (post-centrifugation) and post-thawed cryopreserved PBSCs.
|
Parameter* |
Pre-storage |
Post-thawing |
P value |
|
CD34+ cells (×106 cells/kg) |
0.45 (0.14–1.52) |
0.30 (0.16–1.39) |
> 0.05 |
|
CD34+ cell loss (×106 cells/kg, %) |
0.07 (0.02–0.25), 15.05 (9.73–25.58) |
||
|
CD34+/CD38- cells (×106 cells/kg) |
0.16 (0.07–0.57) |
0.013 (0.06–0.62) |
> 0.05 |
|
CD34+/CD38- cell loss (×106 cells/kg, %) |
0.04 (0.01–0.08), 30.17 (3.91–41.02) |
||
|
TNC count (×108 cells/kg) |
3.32 (2.87–4.82) |
3.24 (2.47–4.49) |
< 0.05 |
|
TNC count loss (×108 cells/kg, %) |
0.27 (0.11–0.48), 8.46 (4.54–12.72) |
||
|
%TNC recovery |
91.54 (87.29–95.46) |
||
Note: All data are shown as median and interquartile intervals (Q1–Q3) of cells.
Percentage of cell viability after deep-freezing and determination by trypan blue exclusion and 7-AAD via flow cytometry
Cell viability after deep-freezing in liquid nitrogen were determined by trypan blue exclusion and compared with those of 7-AAD assessed via flow cytometry. The pre-storage and post-thawing cell viabilities were also determined and compared. The results showed that the viable pre-storage cells were 97.71% (95.25–98.49%) and 98.68% (95.85–99.25%) using trypan blue and 7-AAD, respectively (Table 3). After thawing, the viable cells were 69.44% (59.63–81.13%) and 86.99% (70.50–88.60%) when determined via trypan blue exclusion and flow cytometry, respectively (Table 3). The cell viability percentage significantly decreased by 28.93% and 11.85%, respectively, compared with the pre-storage percentage (P < 0.001).
Table 3. Percentage of cell viability (TNCs) after deep freezing determined by trypan blue exclusion and 7-AAD determined via flow cytometry.
|
Cell viability |
Pre-storage |
Post-thawing |
P value |
|
Trypan blue exclusion (%) |
97.71 (95.24–98.49) |
69.44 (59.63–81.13)** |
< 0.001 |
|
Flow cytometry (7-AAD) (%) |
98.64 (95.85–99.25) |
86.99 (70.50–88.60)** |
< 0.001 |
Note: All data are shown as median and interquartile intervals (Q1−Q3) with a significant difference of **P < 0.001 compared to pre-storage, n = 23.
Effect of deep-freezing cryopreservation on colony-forming units (CFUs) of progenitor and committed cell growth
We next evaluated whether deep-frozen cryopreserved CD34+ cells in liquid nitrogen could grow in the methylcellulose cultures with cytokine cocktails. CD34+ cells caused various types of myeloid colonies including CFU-granulocyte/ erythrocyte/monocyte/megakaryocyte (CFU-GEMM), CFU-granulocyte/monocyte (CFU-GM), CFU-granulocyte (CFU-G), CFU-monocyte (CFU-M), burst-forming unit erythroid (BFU-E), and CFU-erythroid (CFU-E) (Figure 1). BFU-E had the highest colony counts in both pre-storage and post-thawing, followed by CFU-G, then CFU-GM. The post-thawing colony counts demonstrated no significant difference compared with the pre-storage as shown in Table 4 and Figure 2.
Table 4. Colony-forming unit (CFU) counts of progenitor and committed cell growth before deep-freezing and after thawing from cryopreservation.
|
CFU |
Pre-storage (%) |
Post-thawing (%) |
P value |
|
CFU-GEMM |
1.00 (0.33–1.33) |
1.00 (0.67–1.33) |
> 0.05 |
|
CFU-GM |
5.00 (2.50–8.67) |
4.00 (0–9.00) |
> 0.05 |
|
CFU-G |
33.33 (25.00–41.33) |
35.33 (0–46.34) |
> 0.05 |
|
CFU-M |
1.67 (0.83–2.33) |
1.33 (1.00–1.67) |
> 0.05 |
|
CFU-E |
0 (0–0.33) |
0 (0–0.33) |
> 0.05 |
|
BFU-E |
47.67 (20.83–65.83) |
57.67 (39.67–65.83) |
> 0.05 |
|
Total (colonies/1.0×106 cells) |
0.28 (0.14–0.81) |
0.27 (0.13–0.77) |
> 0.05 |
|
%CFU recovery |
94.74 (90.14–95.81) |
|
|
Note: All data are shown as median and interquartile intervals (Q1–Q3) of % CFU, n = 23
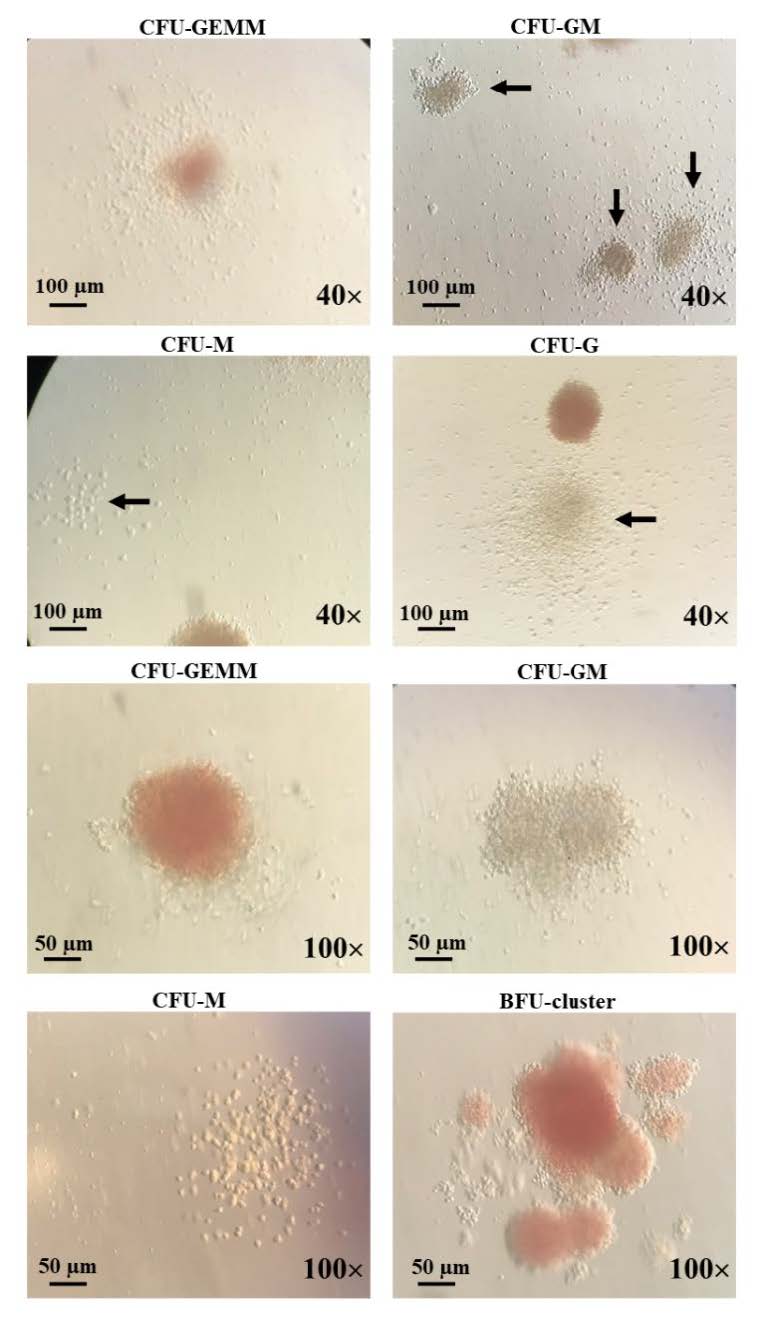
Figure 1. Characteristics of colony-forming units of progenitor cell growth post-thawing from cryopreservation. The ability and potency of the PBSCs were determined by CFUs. The various types of CFUs including CFU-GEMM, CFU- GM, CFU-M, CFU-G, CFU-E, and BFU-E. Bar = 100 μm with 40x magnification and bar = 50 μm with 100x magnification.
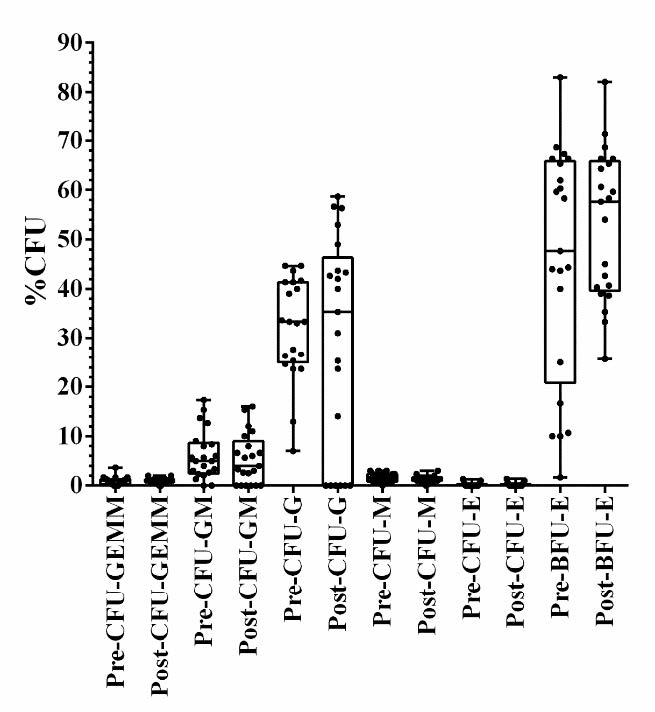
Figure 2. Colony-forming unit (CFU) counts of progenitors and committed cell growth before deep-freezing and after thawing from cryopreservation.
Evaluation of deep-freezing cells on clinical transplantation and engraftment in lymphoma patients
To evaluate deep-freezing cell viability, HSCs from 10 patients (23 samples) were transplanted to the lymphoma patients. All of the bags from the leukapheresis procedures were infused autologously into each patient (100%). The total CD34+ cells were 4.45×106 (2.05–16.25×106)/kg infused patient (CD34+/CD38- cell number was ~1.6×106/kg). After infusion, the absolute neutrophil count (ANC) and platelets were engrafted within 11.00 (10.00–11.50) and 11.00 (10.00–12.00) days (Table 5). The ANC was more than 0.5×109/L and platelet counts were more than 20×109/L for 3 consecutive days without transfusion support. There were no graft failures in any cases.
Table 5. Clinical transplantation and engraftment in lymphoma patients after deep-freezing.
|
Leukapheresis procedures (bag) |
Total CD34+cells (Í 106/kg infused) |
PBSC infused (bag) |
Engraftment |
|
|
ANC (day) |
Platelet (day) |
|||
|
1.50 (1.00–3.50) |
4.45 (2.05–16.25) |
1.50 (1.00–3.50) |
11.00 (10.00–11.50) |
11.00 (10.00–12.00) |
Note: All data are shown as median and interquartile intervals (Q1–Q3), n = 10 patients.
DISCUSSION
Leukapheresis is a laboratory procedure in which white blood cells are separated from a blood sample. CD34+ cells were collected from the lymphoma patients after chemotherapeutic treatments and G-CSF stimulation, acquiring an average number of 0.45×106 (0.14–1.52×106) cells/kg. Deep-freezing cryopreservation is the conventional method for hematopoietic stem cell (HSC) storage and a critical part of hematopoietic stem cell transplantation. Cellular therapy products such as PBSCs may need to be cryopreserved until their use. There are two major methods of cell cryopreservation: deep-freezing and storage in liquid nitrogen at < -180°C and freezing at -80°C. Deep-freezing method is recommended for long-term storage while freezing at -80°C lacks safety evidence. However, freezing at -80°C has been reported to be used for storage for years in a region with limited resources (Shima et al., 2015). The recovery of PBSCs frozen at -80°C versus deep-freezing cryopreservation (-170°C) for 3 and 6 months showed that storage at -80°C had significant loss of cell membrane integrity and clonogenic potential when compared with -170°C (Sputtek et al., 2005; Hornberger et al., 2019). The recovery of viable CD34+ cell populations after deep-freezing was 91%. This result shows the same pattern of previous reports where the recovery of nucleated cells and viable CD34+ cells for adult (n=51) stem cell collections were 83 and 91%, respectively (Sartor et al., 2005; Bekadja et al., 2012). There has not been a previous report about the cell viability and engraftment success of CD34+/CD38- cells after deep-freezing and thawing.
This study focused on the subpopulation of CD34+/CD38- cells (HSCs), and the total cell number of HSCs in PBSCs before and after deep-freezing cryopreservation, assessed using flow cytometry. This is the first report to specify the viability of CD34+/CD38- cells after preservation. The number of CD34+/CD38- cells after collection was 0.16×106 (0.07–0.57×106) cells/kg; 35.56% of the total CD34+ cells, and 0.05% were TNCs. After thawing CD34+/CD38- cell number did not show significant differences compared with that of pre-storage (P > 0.05). CD34+/CD38- cell loss was 30.17% (3.91–41.02%). However, cell viability (conducted using trypan blue exclusion and flow cytometry) showed significant difference compared with the pre-storage (P < 0.05), with values of 28.93% and 11.85%, respectively. These results suggested that other nucleated cells (not including the CD34+ or CD34+/CD38- cells) were dead after thawing. This was observed in TNCs after thawing and significantly decreased (P < 0.05) by 8.46% (4.54–12.72%). CD34+/CD38- cells and CD34+ (HSCs and progenitor cells) cells seem to be fully adaptable compared with other nucleated cells (Pasha et al., 2017). CD34+ cells tolerate up to 60 min exposure to 25% w/w (3.2 M) DMSO at +2°C with no significant loss in clonogenic capacity (Hunt et al., 2003). Different cell types tolerated various ranges of solution osmolarities that are affected by osmotic shocks, which is evidenced by increased necrosis in neutrophils and apoptosis in monocytes. Mature myeloid cells are more sensitive to osmotic stress than lymphocytes and CD34+ cells. Moreover, CD34+/CD38- cells appeared more resistant to cryoinjuries than their CD34+/CD38+ cells, including common myeloid progenitor (CMP) cells, megakaryocyte-erythroid progenitor (MEP) cells, granulocyte/monocyte progenitor (GMP) cells, and common lymphoid progenitor (CLP) cells (Ojeda-Uribe et al., 2004; Woods et al., 2009; Pasha et al., 2017). HSCs from 40 bone marrow and peripheral blood samples were previously reported to be successfully preserved for long-term cryostorage (5–14 years) using a standard in vitro method. Overall, 40% of harvests had CD34+ cell counts of at least 0.7×106/kg and 85% had CFU-GM counts of at least 1.0×105/kg (Spurr et al., 2002).
The potency of CD34+ cells was assessed by CFU assay in a semi-solid medium to compare the number of CFUs and colony types (Akashi et al., 2000; Pamphilon et al., 2013; Morgenstern et al., 2016). The result showed that all of the CFU counts after thawing did not significantly differ from cell growth compared with pre-storage (P > 0.05). The percentage of CFU recovery before storage was 94.74% (90.14–95.81%). The recovery of CFU-GM after thawing was 80%, close to the previous result from human cord blood of 85% (Broxmeyer et al., 2003). Although the CFU results could not be specific to the CD34+/CD38- cell population, the data after thawing CD34+/CD38- cells demonstrated no significant difference compared with pre-storage (P > 0.05). Thus, this result suggests that CD34+/CD38- cells were still alive and demonstrated their potency of cell growth in the CFU assay. In this study, we assessed the quality and recovery of PBSCs as a function of the storage process to improve autologous HSCT treatment for lymphoma. The remainder of the total CFU after deep-freezing and thawing confirmed the tolerance and robust recovery of the CD34+/CD38- cells. The pooled PBSC sample (1.5 [1.00–3.50] bags) with a total of CD34+ cells 4.45–106/kg was infused into the patient. The engraftments of deep-freezing cells were 100% successful within 11 days without graft failure. Absolute neutrophil count (ANC) and platelet engraftment are one of the important outcomes in stem cell transplantation. The engraftment showed the successful of stem cell viability by stem cell collection and preservation. The ANC should be greater than 0.5×109/L (Wolff, 2002) while the platelet count should be more than 20×109/L for 3 consecutive days without transfusion support (Teltschik et al., 2016). Inada, 2001 reported that 22 of the 33 children had received peripheral blood stem cell transplantation using cryopreserved cells. The number of infused CFU-GMs in the PBSCs was 6.9–114.6×104/kg. The cryopreservation time of infused PBSCs was 1–35 months. The ANC achieved 500/mm3 between 8–16 (median 10.5) days, and platelet count achieved 5.0×104/mm3 between 13–200 (median 29) days. This previous report shows the successful engraftment using PBSCs after 35 months cryopreservation (Inada, 2001). There are some limitations in our study. The number of enrolled patients is quite small; however, this study focused on the experiments of stem cell preservation and viability. This study provided a result using human data of engraftment. Further study on animal models and a greater number of patients would be of interest.
CONCLUSION
Leukapheresis has been used to collect and separate the peripheral blood stem cells from lymphoma patient groups and transplant to those patients who have been recommended to replace their bone marrow with their own HSCs (autologous-HSCT). In this study, the storage process was assessed for high-quality recovery of PBSCs for autologous hematopoietic stem cell (HSC) transplantation in lymphoma patients. In conclusion, this study showed a novel finding for CD34+/CD38- cells separation and preservation from PBSCs for autologous HSC transplantation in lymphoma patients by deep-freezing cryopreservation.
ACKNOWLEDGMENTS
We thank the Center for Research and Development of Natural Products for Health, Chiang Mai University, Chiang Mai, Thailand, for chemical, material, and instrumental supports.
AUTHOR CONTRIBUTIONS
Songyot Anuchapreeda, Sawitree Chiampanichayakul, Phennapha Klangsinsisrikul, and Adisak Tantiworawit: conception and study design. Banphot Shaengkhamnang, Pawaret Panyajai, and Adisak Tantiworawit: data acquisition. Banphot Shaengkhamnang, Pawaret Panyajai, Sawitree Chiampanichayakul, Singkome Tima, Phennapha Klangsinsisrikul, Adisak Tantiworawit, and Songyot Anuchapreeda: data analysis and interpretation. Songyot Anuchapreeda, Adisak Tantiworawit, Preeyanat Vongchan, Banphot Shaengkhamnang, and Pawaret Panyajai: drafting or revising the manuscript. All the authors approved of the final article.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
Akashi, K., Traver, D., Miyamoto, T., and Weissman, I.L. 2000. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature. 404: 193-197.
Ali, N., Adil, S.N., and Shaikh, M.U. 2015. Autologous hematopoietic stem cell transplantation—10 years of data from a developing country. Stem Cells Translational Medicine. 4: 873-877.
Araújo, A.B., Salton, G.D., Angeli, M.H., Furlan, J.M., Schmalfuss, T., and Röhsig, L.M. 2022. Effects of cell concentration, time of fresh storage, and cryopreservation on peripheral blood stem cells: PBSC fresh storage and cryopreservation. Transfusion and Apheresis Science. 61: 103298.
Bekadja, M.-A., Brahimi, M., Osmani, S., Arabi, A., Bouhass, R., Yafour, N., Entasoltan, B., Rasheed, W., and Attaf, F. 2012. A simplified method for autologous stem cell transplantation in multiple myeloma. Hematology/Oncology and Stem Cell Therapy. 5: 49-53.
Berens, C., Heine, A., Muller, J., Held, S.A., Mayer, K., Brossart, P., Oldenburg, J., Potzsch, B., Wolf, D., and Ruhl, H. 2016. Variable resistance to freezing and thawing of CD34-positive stem cells and lymphocyte subpopulations in leukapheresis products. Cytotherapy. 18: 1325-1331.
Broxmeyer, H.E., Srour, E.F., Hangoc, G., Cooper, S., Anderson, S.A., and Bodine, D.M. 2003. High-efficiency recovery of functional hematopoietic progenitor and stem cells from human cord blood cryopreserved for 15 years. Proceedings of the National Academy of Sciences. 100: 645-650.
Castelhano, M.V., Reis-Alves, S.C., Vigorito, A.C., Rocha, F.F., Pereira-Cunha, F.G., De Souza, C.A., and Lorand-Metze, I. 2013. Quantifying loss of CD34+ cells collected by apheresis after processing for freezing and post-thaw. Transfusion and Apheresis Science. 48: 241-246.
Donmez, A., Yilmaz, F., Soyer, N., Cagirgan, S., Arik, B., and Tombuloglu, M. 2014. The loss of CD34+ cells in peripheral hematopoietic stem cell products cryopreserved by non-controlled rate freezing and stored at −80°C after overnight storage. Transfusion and Apheresis Science. 51: 188-192.
Facchin, G., Savignano, C., Battista, M.L., Isola, M., De Martino, M., Petruzzellis, G., Rosignoli, C., Pizzano, U., Cerno, M., De Cecco, G. et al. 2022. Impact of cryopreservation of peripheral blood stem cells (PBSC) in transplantation from matched unrelated donor (MUD). Journal of Clinical Medicine. 11: 4114.
Fisher, V., Khuu, H., David-Ocampo, V., Byrne, K., Pavletic, S., Bishop, M., Fowler, D.H., Barrett, A.J., and Stroncek, D.F. 2014. Analysis of the recovery of cryopreserved and thawed CD34+ and CD3+ cells collected for hematopoietic transplantation. Transfusion. 54: 1088-1092.
Hornberger, K., Yu, G., McKenna, D., and Hubel, A. 2019. Cryopreservation of hematopoietic stem cells: Emerging assays, cryoprotectant agents, and technology to improve outcomes. Transfusion Medicine and Hemotherapy. 46: 188-196.
Hunt, C.J., Armitage, S.E., and Pegg, D.E. 2003. Cryopreservation of umbilical cord blood: 2. Tolerance of CD34+ cells to multimolar dimethyl sulphoxide and the effect of cooling rate on recovery after freezing and thawing. Cryobiology. 46: 76-87.
Inada, H. 2001. Cryopreservation and engraftment potential of peripheral blood stem cells: pediatric experience. Kurume Medical Journal. 48: 151-157.
Joint Accreditation Committee - ISCT & EBMT. 2021. International standards for hematopoietic cellular therapy product collection, processing and administration. 8.1th ed[accessed June 27, 2019]. http://www.ebmt.org.
Morgenstern, D.A., Ahsan, G., Brocklesby, M., Ings, S., Balsa, C., Veys, P., Brock, P., Anderson, J., Amrolia, P., Goulden, N. et al. 2016. Post-thaw viability of cryopreserved peripheral blood stem cells (PBSC) does not guarantee functional activity: Important implications for quality assurance of stem cell transplant programmes. British Journal of Haematology. 174: 942-951.
Ojeda-Uribe, M., Sovalat, H., Bourderont, D., Brunot, A., Marr, A., Lewandowski, H., Chaboute, V., Peter, P., and Henon, P. 2004. Peripheral blood and BM CD34+ CD38- cells show better resistance to cryopreservation than CD34+ CD38+ cells in autologous stem cell transplantation. Cytotherapy. 6: 571-583.
Pamphilon, D., Selogie, E., McKenna, D., Cancelas-Peres, J.A., Szczepiorkowski, Z.M., Sacher, R., McMannis, J., Eichler, H., Garritsen, H., Takanashi, M. et al. 2013. Current practices and prospects for standardization of the hematopoietic colony-forming unit assay: A report by the cellular therapy team of the biomedical excellence for safer transfusion (BEST) collaborative. Cytotherapy. 15: 255-262.
Pasha, R., Elmoazzen, H., and Pineault, N. 2017. Development and testing of a stepwise thaw and dilute protocol for cryopreserved umbilical cord blood units. Transfusion. 57: 1744-1754.
Rafiee, M., Abbasi, M., Rafieemehr, H., Mirzaeian, A., Barzegar, M., Amiri, V., Shahsavan, S., and Mohammadi, M.H. 2021. A concise review on factors influencing the hematopoietic stem cell transplantation main outcomes. Health Science Reports. 4: e282.
Sartor, M., Antonenas, V., Garvin, F., Webb, M., and Bradstock, K. 2005. Recovery of viable CD34+ cells from cryopreserved hemopoietic progenitor cell products. Bone Marrow Transplantation. 36: 199-204.
Shima, T., Iwasaki, H., Yamauchi, T., Kadowaki, M., Kiyosuke, M., Mochimaru, T., Takenaka, K., Miyamoto, T., Akashi, K., and Teshima, T. 2015. Preserved in vivo reconstitution ability of PBSCs cryopreserved for a decade at -80°C. Bone Marrow Transplantation. 50: 1195-1200.
Spurr, E.E., Wiggins, N.E., Marsden, K.A., Lowenthal, R.M., and Ragg, S.J. 2002. Cryopreserved human haematopoietic stem cells retain engraftment potential after extended (5–14 years) cryostorage. Cryobiology. 44: 210-217.
Sputtek, A., Rowe, A., and Kuhnl, P. 2005. Long-term storage of peripheral blood progenitor cells at –80°C leads to a pronounced decrease of the clonogenic potential compared to –170°C storage in the vapor phase over liquid nitrogen. Vox Sanguinis. 51: 355.
Sutherland, D.R., Anderson, L., Keeney, M., Nayar, R., and Chin-Yee, I. 1996. The ISHAGE guidelines for CD34+ cell determination by flow cytometry. International Society of Hematotherapy and Graft Engineering. Journal of Hematotherapy. 5: 213-226.
Suwanrungruang, K., Sangrajrang, S., and Buasom, R. 2021. Cancer Incidence in Thailand. In: Rojanamatin J, Ukranun W, Supaattagorn S, Chiawiriyabunya I, Wongsena M, Chiawerawattana A, Laowahutanont P, Chitapanarux I, Vatanasapt P, Greater SL et al., editors [ed]. Cancer in Thailand: Vol X, 2016–2018. Cancer Registry Unit, National Cancer Institute Thailand, Thailand. p. 4-67.
Teltschik, H.M., Heinzelmann, F., Gruhn, B., Feuchtinger, T., Schlegel, P., Schumm, M., Kremens, B., Müller, I., Ebinger, M., and Schwarze, C.P. 2016. Treatment of graft failure with TNI‐based reconditioning and haploidentical stem cells in paediatric patients. British Journal of Haematology. 175: 115-122.
Whitby, A., Whitby, L., Fletcher, M., Reilly, J.T., Sutherland, D.R., Keeney, M., and Barnett, D. 2012. ISHAGE protocol: Are we doing it correctly? Cytometry Part B: Clinical Cytometry. 82B: 9-17.
Wolff, S. 2002. Second hematopoietic stem cell transplantation for the treatment of graft failure, graft rejection or relapse after allogeneic transplantation. Bone Marrow Transplantation. 29: 545-552.
Woods, E.J., Perry, B.C., Hockema, J.J., Larson, L., Zhou, D., and Goebel, W.S. 2009. Optimized cryopreservation method for human dental pulp-derived stem cells and their tissues of origin for banking and clinical use. Cryobiology. 59: 150-157.
Zahid, U., Akbar, F., Amaraneni, A., Husnain, M., Chan, O., Riaz, I.B., McBride, A., Iftikhar, A., and Anwer, F. 2017. A review of autologous stem cell transplantation in lymphoma. Current Hematologic Malignancy Reports. 12: 217-226.
OPEN access freely available online
Natural and Life Sciences Communications
Chiang Mai University, Thailand. https://cmuj.cmu.ac.th
Songyot Anuchapreeda1, 2, *, †, Banphot Shaengkhamnang1, †, Pawaret Panyajai1, Sawitree Chiampanichayakul1, 2, Singkome Tima1, 2, Phennapha Klangsinsirikul1, Preeyanat Vongchan1 and Adisak Tantiworawit3, *
1 Department of Medical Technology, Faculty of Associated Medical Sciences, Chiang Mai University, Chiang Mai 50200 Thailand.
2 Cancer Research Unit of Associated Medical Sciences (AMS-CRU), Faculty of Associated Medical Sciences, Chiang Mai University, Chiang Mai 50200 Thailand.
3 Department of Internal Medicine, Faculty of Medicine, Chiang Mai University, Chiang Mai 50200 Thailand.
Corresponding author: Songyot Anuchapreeda E-mail: songyot.anuch@cmu.ac.th
Adisak Tantiworawi E-mail: atantiwo@med.cmu.ac.th
† These authors contributed equally
to this work.
Total Article Views
Editor: Waraporn Boonchiang,
Chiang Mai University, Thailand
Article history:
Received: February 27, 2022;
Revised: May 12, 2023;
Accepted: May 16, 2023;
Published online: May 26, 2023

